Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Ionic Compounds
Part a: Properties of Ionic Compounds
Part 1a: Properties of Ionic Compounds
Part 1b: Combining Ions to Form Ionic Compounds
Part 1c: Binary Ionic Compounds
Part 1d: Compounds Containing Polyatomic Ions
What is an Ionic Compound?
In Lesson 3 of Chapter 3, the topic of ions was discussed. We looked at the patterns by which main group elements form positive and negative ions. And we discussed transition metal elements that form ions with different charges. Finally, we looked at the topic of polyatomic ions. In this lesson of Chapter 4, we will build on that information to explain what an ionic compound is and how to write the name and formula for such compounds.
 An ionic compound is formed from two ions. A positive ion or cation binds together with a negative ion or anion. As a simple example, let’s consider sodium ions (Na+) and chloride ions (Cl-) forming an ionic compound. A fundamental rule in science is that oppositely charged objects will attract. The opposite charges of Na+ ions and Cl- ions results in a strong electric force that holds the two ions together. We refer to the force as an ionic bond and the compound that results from this bond is called an ionic compound. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions.
An ionic compound is formed from two ions. A positive ion or cation binds together with a negative ion or anion. As a simple example, let’s consider sodium ions (Na+) and chloride ions (Cl-) forming an ionic compound. A fundamental rule in science is that oppositely charged objects will attract. The opposite charges of Na+ ions and Cl- ions results in a strong electric force that holds the two ions together. We refer to the force as an ionic bond and the compound that results from this bond is called an ionic compound. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions.
Crystalline Structure
 One of the most shared properties of ionic compounds is that they are crystalline solids. This property can be explained at the particle level. The positive and negative ions form a tightly packed, three-dimensional arrangement of alternating positively and negatively charged ions. This close packing reduces the distance between ions of opposite charge and maximizes the strength of the attractive forces between those ions. The orderly arrangement of the ions into a structure is referred to as a crystal lattice.
One of the most shared properties of ionic compounds is that they are crystalline solids. This property can be explained at the particle level. The positive and negative ions form a tightly packed, three-dimensional arrangement of alternating positively and negatively charged ions. This close packing reduces the distance between ions of opposite charge and maximizes the strength of the attractive forces between those ions. The orderly arrangement of the ions into a structure is referred to as a crystal lattice.
The diagram below (left) models the crystal lattice structure formed by Na+ ions and Cl- ions. The ions are represented by spheres. The larger green spheres are chloride ions. The smaller purple spheres are sodium ions. Each sodium ion is surrounded by six chloride ions, allowing for multiple attractions with nearby ions of the opposite charge. Similarly, each chloride ion is surrounded by six sodium ions. The strong forces between neighboring ions of opposite charge explains why ionic compounds form crystalline solids.
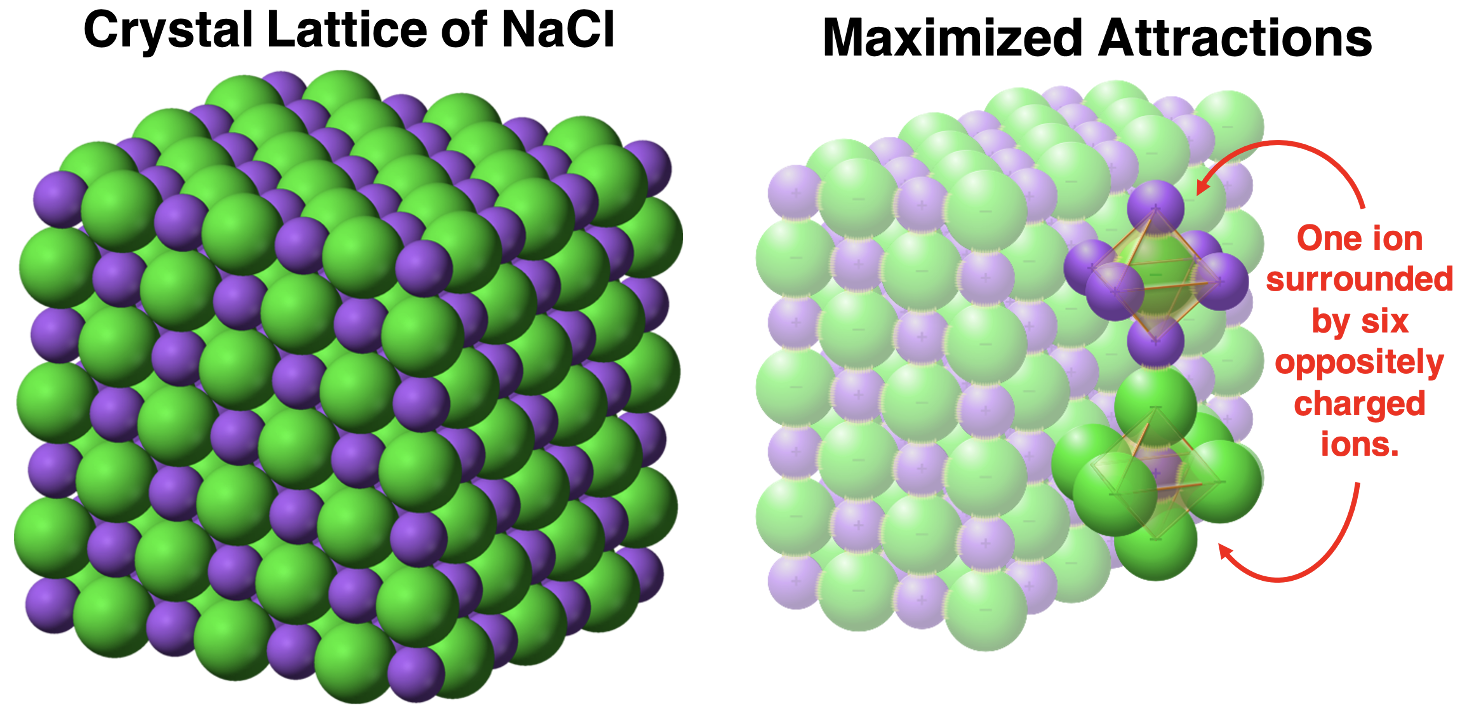
Image Sources: Crystalline Salt || Crystal Lattice of NaCl || Maximized Attractions
High Melting Points
 A particle-level description of the three states of matter was given in Chapter 2 of this Chemistry Tutorial. Solids were characterized by strong forces that pull the particles close together and hold them in a locked position. Particles of a solid are only capable of vibrating about their fixed position. They are not able to move about the bulk of the solid. In contrast, the attractive forces in liquids are too weak to hold particles in a fixed position. Particles of a liquid are in constant motion about the bulk of the liquid.
A particle-level description of the three states of matter was given in Chapter 2 of this Chemistry Tutorial. Solids were characterized by strong forces that pull the particles close together and hold them in a locked position. Particles of a solid are only capable of vibrating about their fixed position. They are not able to move about the bulk of the solid. In contrast, the attractive forces in liquids are too weak to hold particles in a fixed position. Particles of a liquid are in constant motion about the bulk of the liquid.
Melting involves the transition of a sample from the solid state to the liquid state. Increasing the temperature of a solid increases the vigor of the back-and-forth vibrations of the particles. The higher the temperature, the more wildly that the particles of the solid vibrate. Melting occurs when those vibrations are vigorous enough to allow the solid particles to escape the forces that hold them in their fixed position. Once solid particles escape those forces, they are free to move about the bulk of the substance as a freed liquid.
 For ionic compounds, the forces that hold ions in the crystal lattice are abnormally strong. As such, it takes a lot of vibrating for the ions to free themselves from the fixed position. In order to achieve such high levels of particle vibration, higher temperatures are required. As such, ionic compounds have high melting point temperatures. The table at the right compares the melting point temperatures of ionic compounds to that of non-ionic substances.
For ionic compounds, the forces that hold ions in the crystal lattice are abnormally strong. As such, it takes a lot of vibrating for the ions to free themselves from the fixed position. In order to achieve such high levels of particle vibration, higher temperatures are required. As such, ionic compounds have high melting point temperatures. The table at the right compares the melting point temperatures of ionic compounds to that of non-ionic substances.
Electrolyte in Solution
Due to their high melting points, ionic compounds are solids at room temperatures. And as a solid, ionic compounds are not electrical conductors. To be a conductor of electricity, a substance must be able to allow for the flow of charges. The ions of ionic solids are locked into the crystal lattice and unable to flow. But at temperatures above their melting point, ionic compounds assume a liquid state and act as electrical conductors.
It's time for a thought experiment. Let’s suppose we have three beakers of pure water, labeled A, B, and C. We have a conductivity tester that allows us to test if the substance in the beaker is a conductor of electricity. We test the pure water and observe that it does not conduct. Beaker A is our control; we won’t make any changes to it. But we will add sucrose (C12H22O11) to beaker B and sodium chloride (NaCl) to beaker C. The solids are stirred and dissolve in the water. Then we test to see if they conduct electricity. The results are shown below.
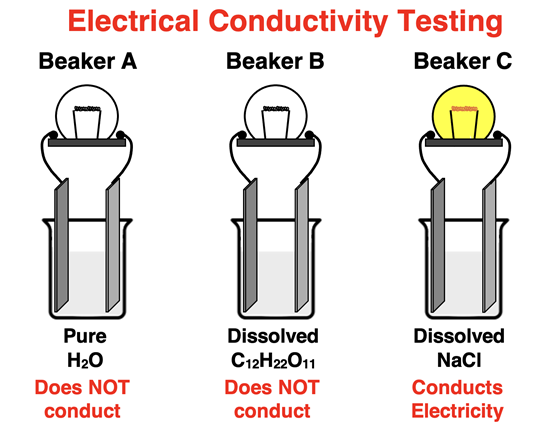
Dissolving sucrose into the water of beaker B did not cause the water’s conductivity to change. But dissolving NaCl into the water of beaker C caused the solution to become a conductor of electricity.
This is typical behavior for ionic solids. When dissolved in water, they become conductors of electricity. Why would that be? Once more, a macroscopic observation can be explained by particle-level behavior. In the solid state, the ions are incapable of conducting electricity because they are locked into a crystal lattice. Dissolving causes the ions to be freed from their fixed position and capable of moving about the bulk of the solution. A change from the solid state to the aqueous state (i.e., dissolved in water) changes the ions from a fixed position to mobile carriers of charge. The mobile ions allow the solution to conduct electricity. We refer to ionic solids are electrolytes. In Chemistry, electrolytes are substances that can conduct electricity when dissolved in water.
Hard and Brittle
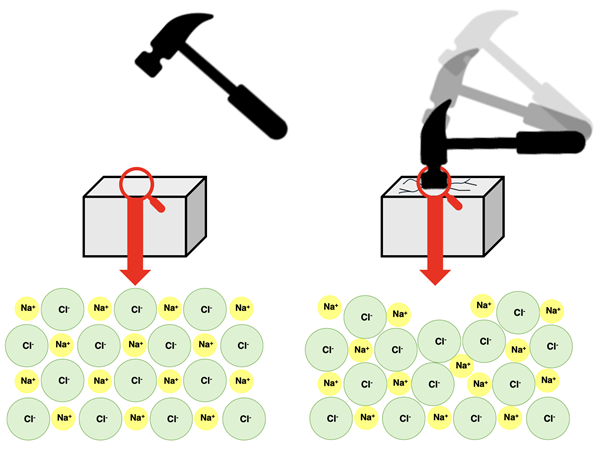
Ionic compounds exhibit the property of hardness. A hard material will tend to assume its shape when stressed by an external force. Hard materials seldom deform from their original shape and contour. Hard materials are often very brittle. And ionic solids exhibit this property brittleness. A brittle material is one that breaks or shatters when a large force or impact is applied to it. Because ionic solids have very little flexibility when impacted, they will tend to shatter or break when a force exceeds some threshold value.
The hard and brittle qualities of an ionic compound are explained by its habit of forming a crystal lattice structure with strong inter-ion forces. In order to withstand a large force without breaking or shattering, particles of the material must have the ability to momentarily slide out of their lattice positions and even past one another. This gives them the ability to deform with the impact and then re-assume their original shape. The close proximity of ions in the lattice and the strong forces between them hinder such motion. When the impact on the surface is large enough, ions will be displaced from their fixed position. Like-charged ions end up getting too close to each other, leading to destructive repulsive forces. The lattice structure is disrupted and never recovers. The surface ends up shattering.
Before You Leave
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Based on what you learned about ions in
Lesson 3 of Chapter 3 and about ionic compounds on this page, which of the following elements would you expect to form ionic compounds? Use a
periodic table.
- Sodium (Na) and potassium (K)
- Potassium (K) and sulfur (S)
- Nitrogen (N) and fluorine (F)
2. Which of the following ions would attract one another to form ionic compounds?
- Sodium ions (Na+) and ammonium ions (NH4+)
- Ammonium ions (NH4+) and sulfide ions (S2-)
- Sulfide ions (S2-) and sulfate ions (SO42-)
- Sulfate ions (SO42-) and ammonium ions (NH4+)
- Sodium ions (Na+) and sulfate ions (SO42-)
3. Identify the following statements about
ionic solids as being either
TRUE or
FALSE.
- Increasing temperatures causes the attractions between ions to become weaker.
- Increasing temperatures causse ions to vibrate more vigorously.
- Increasing temperatures lower the melting point of ionic solids.
- Ionic solids melt at temperatures greater than the melting point.
4. Suppose that the conductivity testing was performed with additional compounds being dissolved in water. Predict the result of the tests for the following compounds. Which would conduct and which would not conduct electricity?
- Ethanol (C2H6O)
- Lithium fluoride (LiF)
- Carbon dioxide (CO2)
- Zinc chloride (ZnCl2)