Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Ionic Compounds
Part d: Compounds Containing Polyatomic Ions
Part 1a: Properties of Ionic Compounds
Part 1b: Combining Ions to Form Ionic Compounds
Part 1c: Binary Ionic Compounds
Part 1d: Compounds Containing Polyatomic Ions
What is a Polyatomic Ion?
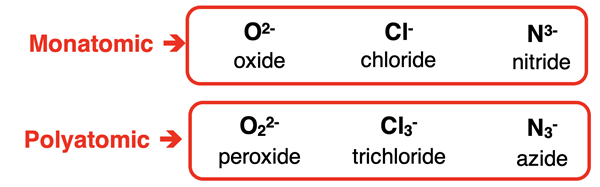
Ions can be monatomic or polyatomic. Monatomic ions like Na+ or O2- consist of a single atom with unequal numbers of protons and electrons. A polyatomic ion consists of two or more atoms packaged together as a single unit. The total number of protons contributed by all the atoms is not equal to the number of electrons, making the particle an ion. Each polyatomic ion has its own unique name. Ion symbols and names are typically listed in a polyatomic ion table. Here are five of many examples of polyatomic ions:

Polyatomic ions contain two or more atoms. Most also contain two or more elements, like the five examples above. There are a small number of polyatomic ions that contain a single element. Examples include the peroxide ion (O22-), the azide ion (N3-), and the trichloride ion (Cl3-). You will need to be aware that these three polyatomic ions have different names and symbols than their monatomic look-alikes.
Ionic Compounds with Polyatomic Ions
Previously, we learned how to write names and formulae for binary ionic compounds. Binary ionic compounds consist of a metal acting as the cation and a nonmetal that acts as the anion. In this part of Lesson 1 we will learn how to write the names and formulas of ionic compounds containing polyatomic ions. This will include ionic compounds in which one or both of the ions are polyatomic. The procedure for naming and formula writing will be similar to that of binary ionic compounds. You will need to make some minor adjustments to accommodate for the polyatomic ion in the compound.
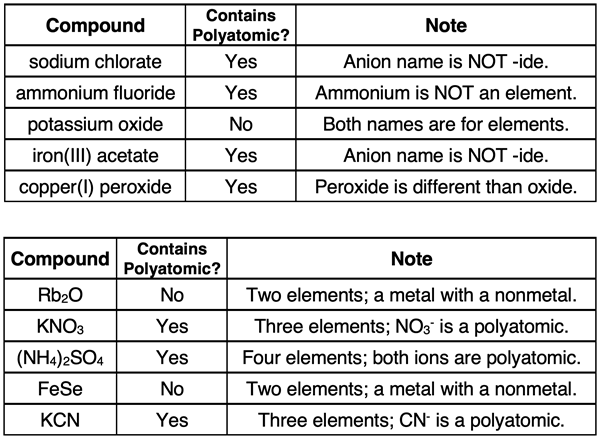
The surest way to identify compounds with a polyatomic ion is to look for the presence of three or more elements. If you see three elements in the formula, then you know that the compound contains at least one polyatomic ion. If a name is given instead of a formula, then carefully inspect the two words in the name. If the first word (representing the cation) is not the name of an element, then it is probably a polyatomic ion. For instance, ammonium is not an element; it is a polyatomic ion. If the second word of the name does not end in -ide, then you can be certain that the anion is a polyatomic ion. You will have to be careful as there are several polyatomic ions that end with -ide. Examples include hydroxide (OH-), cyanide (CN-), peroxide (O22-), triiodide (I3-), etc. Having a periodic table and a polyatomic ion list readily available will help you with the task of identifying the polyatomic ion in an ionic compound. The table below includes several examples and non-examples of ionic compounds that contain polyatomic ions.
Ion Ratios
You have previously learned that formulas for ionic compounds list the symbols for each ion and include subscripts to indicate the relative number of the two ions. While ionic compounds contain charged ions, the overall charge is zero. The sum of all the positive charges must equal the sum of all the negative charges. You can use this principle to determine the ion ratio. We did this earlier in Lesson 1 for binary ionic compounds.  Now we will discuss ion ratios for compounds containing polyatomic ions. (Have a periodic table and a polyatomic ion list readily available as we proceed.)
Now we will discuss ion ratios for compounds containing polyatomic ions. (Have a periodic table and a polyatomic ion list readily available as we proceed.)
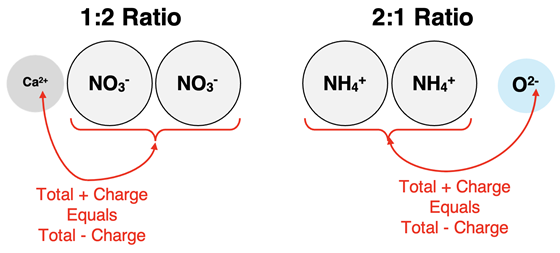
Suppose two ions have the opposite type and the same amount of charge. They will combine with a 1:1 ion ratio in order to form a neutral ionic compound. The subscripts in the formula would show that there are equal numbers of the two ions in a formula unit. They would both have a subscript of 1 (that is, if we used 1s as a subscript).
Suppose one ion has twice the charge of the other ion. The ion ratio would be 1:2 or 2:1. There would need to be twice as many of the lesser-charged ions to balance the twice-larger charge of the other ion. The ion with the lesser charge would have a subscript of 2 in the formula.
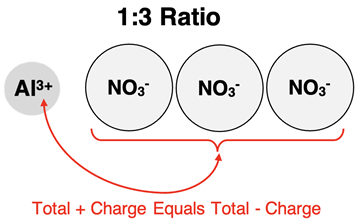
Suppose one ion has triple the charge of the other ion. The ion ratio would be 1:3 or 3:1. There would need to be three times as many of the lesser-charged ions to balance the three-times-larger charge of the other ion. The ion with the lesser charge would have a subscript of 3 in the formula.
Finally, consider the situation in which one ion is 3+ and the other ion is 2-. What is the ratio by which they combine? After trying a variety of possible ratios, you would discover that they would combine in a 2:3 ratio in order for the compound to be neutral. There would need to be a subscript of 3 after the 2- ion and a subscript of 2 after the 3+ ion.
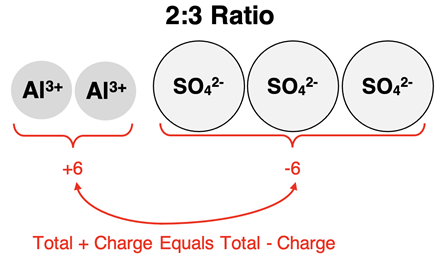
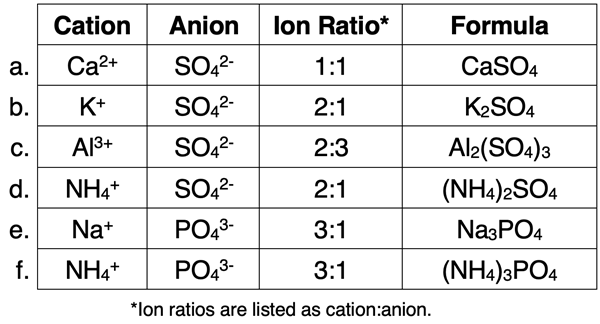
The table below shows several additional examples of ion ratios for compounds containing polyatomic ions. The last column illustrates how the ion ratios are turned into subscripts when writing the formula.
Use of Parentheses
 You may have noticed something new in rows c, d, and f of the above table. The formula for the polyatomic ion is surrounded by parenthesis. This is done whenever there is a subscript of 2 or greater. Parentheses are not used for the monatomic ions. And parentheses are not used if there is no subscript listed after it.
You may have noticed something new in rows c, d, and f of the above table. The formula for the polyatomic ion is surrounded by parenthesis. This is done whenever there is a subscript of 2 or greater. Parentheses are not used for the monatomic ions. And parentheses are not used if there is no subscript listed after it.
 Name to Formula
Name to Formula
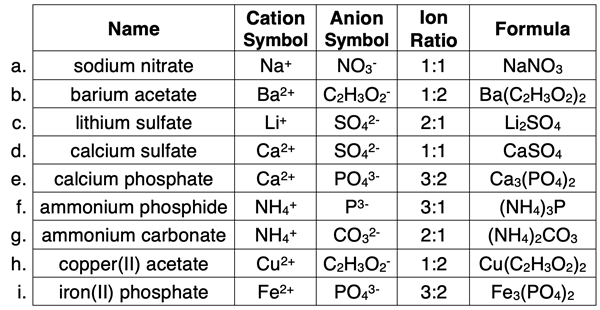
Chemistry students need to learn how to write the formula of an ionic compound if given the name of the ionic compound. The name consists of two words. The first word is the cation (+ ion). The second word is the anion (- ion). Use a periodic table and a polyatomic ion list to determine the symbol and the charge of the cation and the anion. Be careful and give attention to detail. A step-by-step procedure is shown at the right. Several examples are included in the table below. Additional practice ideas can be found in Before You Leave section and the Check Your Understanding section.
 Formula to Name
Formula to Name
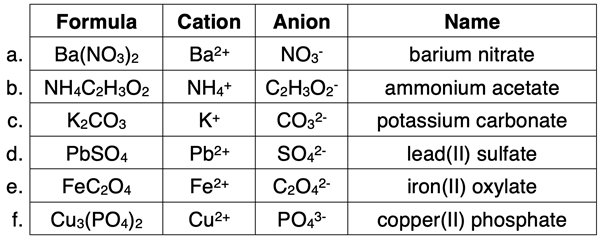
Chemistry students will also need to acquire the skill of writing the name of an ionic compound if given the formula. This was demonstrated for binary ionic compounds on a previous page. Now we will use an almost identical procedure to write the names for ionic compounds containing polyatomic ions. To begin, you will need to inspect the formula and identify the dividing line between the cation and the anion. The cation is always listed first. Most often it is a monatomic ion. Ammonium (NH4+) is the most notable exception. The rest of the formula is the anion. Once you have identified the cation and the anion in the formula, you can write the name. The procedure is shown at the right. Have a periodic table and a polyatomic ion list readily available. Examples are shown in the table below. Additional practice ideas can be found in Before You Leave section and the Check Your Understanding section.
Before You Leave
- If you don't have a polyatomic ion list, consider downloading ours. Download Polyatomic Ion List.
- Download our Study Card on Ionic Compounds with Polyatomic Ions (Names and Formulae). Save it to a safe location and use it as a review tool. (Coming Soon)
- Try our Concept Builder titled Names to Formula 2. The second activity (Master Level) would provide great practice with identifying the formulae for ionic compounds containing polyatomic ions.
- The Check Your Understanding section below include questions and problems with answers and explanations and solutions. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready. Use a
periodic table and a
polyatomic ion list if necessary.
1. Explain
the rule for when you enclose a polyatomic ion in parentheses.
2. What’s Wrong with This?
A teacher is grading a quiz on nomenclature. She sees the following formulas. Identify what is wrong with each formula.
- copper(II) nitrite → Cu(NO3)2
- sodium sulfite → Na2SO4
- calcium nitrate → CaNO3
- calcium carbonate → Ca(CO3)
- iron(III) acetate → Fe3C2H3O2
- lithium sulfate → (Li)2SO4
3. And What’s Wrong with This?
A teacher is grading a quiz on nomenclature. He sees the following names of ionic compounds. Identify what is wrong with each name.
- Na2SO4 → sodium hydrogen oxide
- Na2SO4 → sodium sulfate(IV)
- KNO2 → potassium nitrate
- BaSO4 → barium(II) sulfate
- CuSO4 → copper sulfate
- Cu(NO3)2 → copper(I) nitrate
4. Determine the names associated with the following formulas.
- K2SO3 →
- K2SO4 →
- K2S →
- CuCO3 →
- Cu(HCO3)2 →
- FePO4 →
- Pb(C2H3O2)2 →
- Sn(NO3)4 →
- (NH4)3P →
5. Determine the formulas associated with the following names.
- sodium nitrite →
- magnesium nitrite →
- sodium nitride →
- sodium nitrate →
- ammonium carbonate →
- iron(II) carbonate →
- ammonium sulfide →
- ammonium carbonate →
- aluminum sulfate →
- sodium peroxide →