Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Matter and Its Classification
Part a: Solids, Liquids, and Gases
Part 1a: Solids, Liquids, and Gases
Part 1b: Pure Substances vs. Mixtures
Matter
Earlier in Chapter 1, we briefly discussed the topic of matter. Matter refers to the stuff that is all around us. It includes the everyday materials, the water that we drink, the air that we breathe, and your friends, families, and pets. This stuff we call matter is defined as anything that has mass and occupies space. Essentially, anything that exists can be identified as matter with the exception of those phenomenon that we classify as waves. Waves, like sound waves and light waves, do not have mass and do not occupy space. Waves may move through matter; but waves are not matter. Waves are waves; everything else is matter. The nature of waves is a topic more commonly discussed in a Physics class. In Chemistry, our focus is on the study of matter.
Our goal in this chapter of our Chemistry Tutorial is to discuss the manner in which chemists describe matter and how one sample of matter can be distinguished from another sample of matter. If you’re given a sample of stuff, how would you describe it? If you were to tell a friend about it, what characteristics would you point out that are descriptive of this stuff? Perhaps one of the more obvious characteristics might be whether the stuff is a solid, a liquid, or a gas.
Three States of Matter
 A sample of matter can be classified as being either a solid, a liquid, or a gas. These three states of matter are distinguished from each other by their observable properties and the underlying particle-level characteristics. A solid has a fixed shape and a fixed volume. Your pencil is an example of a solid object. It’s shape will remain the same no matter what room you put it in. It’s volume – the amount of space it occupies – will also be the same regardless of the container that it is in.
A sample of matter can be classified as being either a solid, a liquid, or a gas. These three states of matter are distinguished from each other by their observable properties and the underlying particle-level characteristics. A solid has a fixed shape and a fixed volume. Your pencil is an example of a solid object. It’s shape will remain the same no matter what room you put it in. It’s volume – the amount of space it occupies – will also be the same regardless of the container that it is in.
 In contrast to a solid, a liquid has a variable shape and a fixed volume. Water poured from a faucet is a liquid. Suppose you poured 250 mL of water from the faucet into a large glass from the cupboard. The shape assumed by the water is dependent upon the container that it is in. If the 250 mL of water were poured from the large glass into a small baking pan, it would assume a different shape. But there would still be 250 mL of water in the baking pan. The volume of the water is fixed but its shape is variable since shape is dependent upon the container that holds the water. These characteristics of liquid water are quite different than that of the solid pencil. Being a solid, the pencil’s shape and volume are fixed and independent of the container that it is in.
In contrast to a solid, a liquid has a variable shape and a fixed volume. Water poured from a faucet is a liquid. Suppose you poured 250 mL of water from the faucet into a large glass from the cupboard. The shape assumed by the water is dependent upon the container that it is in. If the 250 mL of water were poured from the large glass into a small baking pan, it would assume a different shape. But there would still be 250 mL of water in the baking pan. The volume of the water is fixed but its shape is variable since shape is dependent upon the container that holds the water. These characteristics of liquid water are quite different than that of the solid pencil. Being a solid, the pencil’s shape and volume are fixed and independent of the container that it is in.
 Gases have their own unique properties. A gas has a variable shape and a variable volume. The air that you breathe is a gas. Suppose you take a deep breath and inhale air from the room. The air would enter your lungs and expand to fill your lungs, occupying the space in your lungs along with the air that was already there. Since the sample of air you inhaled fills your entire lungs, its shape would be the shape of your lungs. Its volume would be the volume of your lungs, estimated at roughly 5 liters. Now suppose you could exhale that same sample of air into a balloon. The air would fill the balloon and take on a new shape, the shape of the balloon. Its volume would likely be considerably less, roughly estimated at 0.5 liters (based on the volume of an average inhaled and exhaled breath). You will notice that the shape of the air sample is variable and depends on the container. The volume of the air sample is also variable. Since gases like air expand to fill the entire container, the volume of air is also dependent upon the container that holds it.
Gases have their own unique properties. A gas has a variable shape and a variable volume. The air that you breathe is a gas. Suppose you take a deep breath and inhale air from the room. The air would enter your lungs and expand to fill your lungs, occupying the space in your lungs along with the air that was already there. Since the sample of air you inhaled fills your entire lungs, its shape would be the shape of your lungs. Its volume would be the volume of your lungs, estimated at roughly 5 liters. Now suppose you could exhale that same sample of air into a balloon. The air would fill the balloon and take on a new shape, the shape of the balloon. Its volume would likely be considerably less, roughly estimated at 0.5 liters (based on the volume of an average inhaled and exhaled breath). You will notice that the shape of the air sample is variable and depends on the container. The volume of the air sample is also variable. Since gases like air expand to fill the entire container, the volume of air is also dependent upon the container that holds it.
The contrasting properties of the three states of matter are summarized in the table.

Now someone may be thinking, But what about …? If you are, then you should be praised for being well-read … and also for keeping an active mind as you are reading. You’re probably thinking that there are more than three states of matter. And you’re right. There’s a fourth and a fifth state of matter. Matter can also exist as a plasma and as a Bose-Einstein condensate. These two states of matter exist under more extreme conditions. A plasma state is achieved when a gas is heated to even higher temperatures, resulting in the freeing of electrons from the gas particles and the formation of a soup of charged particles. And a Bose-Einstein condensate is a state of matter achieved at temperatures very close to 0 K (-273°C); it is characterized by subatomic particles clumping together in the same quantum energy state. These two additional states are upper-level topics. Keep moving forward in your science studies and someday you can explain them to us.
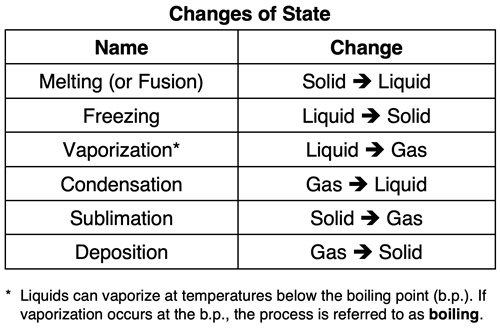
Changes of State
A sample of matter can be changed from one state to another state. Heating, cooling, and/or changes in pressure can cause these state changes. The transitions or changes between states are processes that have specific names. The table below shows the names that are given for the various changes of state.
Particle Level Explanations
 Often times in Chemistry, the observations that we make reveal something more fundamental about matter at the particle level – on the level of atoms and molecules. The macroscopic observations – the things we observe - are indicators of particle-level or particulate realities. This connection between the observables and the particulate is very obvious when it comes to a comparison of solids, liquids, and gases. Many of the properties that make a solid a solid, a liquid a liquid, and a gas a gas are explained by how particles in the sample behave.
Often times in Chemistry, the observations that we make reveal something more fundamental about matter at the particle level – on the level of atoms and molecules. The macroscopic observations – the things we observe - are indicators of particle-level or particulate realities. This connection between the observables and the particulate is very obvious when it comes to a comparison of solids, liquids, and gases. Many of the properties that make a solid a solid, a liquid a liquid, and a gas a gas are explained by how particles in the sample behave.
In the solid state, particles in a sample have strong attractive forces for one another. These strong forces pull the particles close together and hold them in a locked position. This is what gives solids a fixed shape and a fixed volume. Solids don’t flow like liquids and gases because of these strong forces and locked particle positions. Unlike liquids and gases, you can grasp a solid object with your hand. And you can move the solid to another location. No matter what container the solid is placed in, its shape and volume will not change since the strong inter-particle forces hold the particles of the sample together.
The gaseous state lies on the opposite end of the state continuum. There is nearly no attractive forces between particles in the gas state. Because of this, gas particles do not occupy a fixed position but instead expand to fill the container. This explains their variable volume and variable shape.
 The liquid state can be thought of as the intermediate state between solids and gases. There are attractive forces between particles in the liquid state; but they are rather weak forces when compared to solids. They are incapable of holding liquid particles in a fixed position. Thus, liquids exhibit the property of being able to flow. Their shape is thus determined by the shape of the container that holds the liquid. While the forces are too weak to hold particles in a fixed position, they are strong enough to prevent particles from leaving the herd and expanding to fill the entire container. Particles still hang out together and thus their volume is fixed and not dependent upon the container that they are in.
The liquid state can be thought of as the intermediate state between solids and gases. There are attractive forces between particles in the liquid state; but they are rather weak forces when compared to solids. They are incapable of holding liquid particles in a fixed position. Thus, liquids exhibit the property of being able to flow. Their shape is thus determined by the shape of the container that holds the liquid. While the forces are too weak to hold particles in a fixed position, they are strong enough to prevent particles from leaving the herd and expanding to fill the entire container. Particles still hang out together and thus their volume is fixed and not dependent upon the container that they are in.
The above discussion explains the connection between what is happening at the particle level (the particulate) and the properties we observe (the macroscopic). This will be a common theme in Chemistry as we seek to build models of particle behavior to explain the world that we observe and experience.
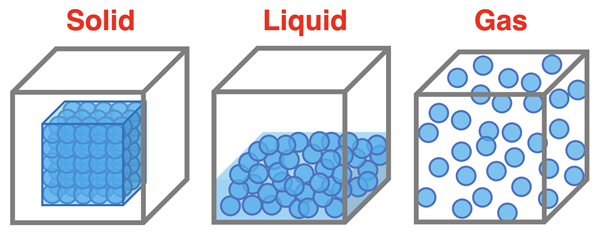
Particle Diagrams
Particle diagrams are a commonly used tool to assist in thinking about matter at the particle level. Particle diagrams represent the particles of a sample of matter by small circles. The particles can be thought of as atoms or molecules or ions; we will have much more to say about these in Chapter 3. The particle diagrams below are representations of the three states of matter. The solid-state diagram depict an orderly arrangement of close-together particles occupying a fixed position. The sample does not expand to fill the container. There is a fixed volume and a fixed shape. The gaseous state diagram depicts rather distant particles with no ordered arrangement that fill the entire volume of the container and assume the shape of the container. The liquid state depicts close-together molecules with no orderly arrangement with a shape that is determined by the container and occupying a volume that is not determined by the container.
Before You Leave
- It's a good thing to read about Chemistry. But it is an even better thing to put to practice the ideas that you have read about. Consider some practice – try our States of Matter Concept Builder.