Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Ionic Compounds
Part b: Combining Ions to Form Ionic Compounds
Part 1a: Properties of Ionic Compounds
Part 1b: Combining Ions to Form Ionic Compounds
Part 1c: Binary Ionic Compounds
Part 1d: Compounds Containing Polyatomic Ions
Ionic Compounds are Neutral
As discussed
previously in Lesson 1, ionic compounds are formed by the electrical attraction between a positive ion (cation) and a negative ion (anion). While each ion is charged, the overall compound is electrically neutral. As a rule, compounds have no net charge. For this to be true, the oppositely charge ions must combine in such a manner that the contribution of positive charge by the cation balances the contribution of negative charge by the anion. This means that the ions may combine in a 1:1 ratio, a 2:1 ratio, a 3:1 ratio, a 3:2 ratio, etc. The ratio by which the ions combine to form the neutral compound is affected by the charges on the ions.
Ion Charge
In
Chapter 3, we discussed
ions formed from main group elements and
ions formed from transition metals. We also discussed
polyatomic ions. We learned that main group metals and nonmetals form ions with very predictable charges. The predictable charges of Groups 1, 2, and 13 metals are shown below. The predictable charges of Groups 15, 16, and 17 nonmetals is also shown.
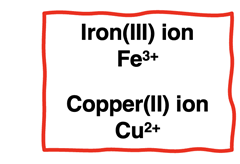
Transition metals are less predictable. Transition metals will form positive ions. But these elements can form multiple ions with different charges. For instance, iron can form ions with a 2+ or a 3+ charge The charge of the ion is indicated by
a Roman numeral enclosed in parenthesis. An iron(III) ion has a 3+ charge.
Finally, many anions are
polyatomic ions. There are even a few polyatomic cations. A
polyatomic ion is a collection of atoms bound together as a single bundle; the bundle of atoms has an overall charge. The sulfate ion, SO
42-, is an example of a polyatomic ion. The nitrate ion, NO
3-, is another example. Learning the names and formulas of polyatomic ions can be quite overwhelming. A summary of the most common polyatomic ions can be found in our Reference section.
Knowing the charge on an ion will be critical for acquiring the skill of determining the ratio by which two ions will combine to form a neutral ionic compound.
Determining Ion Ratios
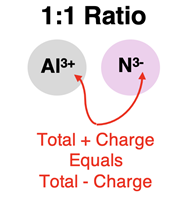
One important skill that a Chemistry student must acquire is the skill of identifying the simplest ratio by which the cation and anion combine to form ionic compounds. This ratio is referred to as the
ion ratio. If the two combining ions have identical amounts of charge (for instance, 2+ with 2-), then they combine with a 1: 1 ion ratio. If there are equal numbers of Ca
2+ combining with O
2-, then the positive charge will balance the negative charge and the compound will be overall neutral. Similarly, Al
3+ will combine with N
3- with a 1:1 ratio to form a neutral ionic compound.
If one ion has twice the amount of charge (but opposite type) as the other ion, then they will combine in a 1:2 or a 2:1 ratio. For instance, a Ca
2+ ion has twice the amount of charge as a Cl
- ion. So, there must be two times as many Cl
- ions than Ca
2+ in the compound in order for the amount of negative charge to balance the amount of positive charge. Ca
2+ ions will combine with Cl
- ions in a 1:2 ion ratio in order to have a net neutral compound.
If one ion has three times the amount of charge (but opposite type) as the other ion, then they will combine in a 1:3 or a 3:1 ration. For instance, a Al
3+ ion has three times the amount of charge as a Cl
- ion. So, there must be three times as many Cl
- ions than Al
3+ in the compound in order for the amount of negative charge to balance the amount of positive charge. Al
3+ ions will combine with Cl
- ions in a 1:3 ion ratio in order to have a net neutral compound.
Now suppose that we have an Al
3+ ion combining with a O
2- ion. By what ion ratio would they combine to form a neutral ionic compound? Determining the answer takes a bit more thought. Be governed by the rule that the ions combine in a ratio such that the amount of positive charge balances the amount of negative charge. The simplest ratio of ions would be a 2:3 ratio. Two ions of Al
3+ combine with three ions of O
2-.The six units of positive charge from the two aluminum ions will equal the six units of negative charge from the three oxide ions.
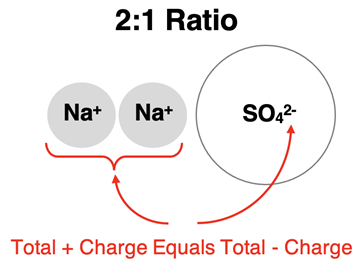
The practice of determining ion ratios can be applied to polyatomic ions in the same manner. Suppose an ionic compound is formed between Na
+ ions and SO
42- ions. The amount of charge on a sodium ion is one-half the amount of charge on a sulfate ion. There must be twice as many sodium ions as sulfate ions in order for the amount of positive charge to equal the amount of negative charge. So, Na
+ ions and SO
42- ions form an ionic compound by combining in a 2:1 ratio.
The Overarching Principle … and a Bit of Preaching
It is important in Chemistry that concepts be internalized and not memorized. Always make an effort to reduce the amount of information stored in memory. Replace memorizing with processing. Increase the power of your processor to analyze multiple and varied situations while being guided by an overarching principle. To do this you will need to put energy into making meaning of ideas. Much of the
meaning-making can be done by utilizing our
Check Your Understanding sections and the links we provide to interactive website exercises in the
Before You Leave sections. Any student can memorize the ion ratios for specific ion charges. But that’s too much to memorize. Instead, remember and learn how to use the overarching principle:
Oppositely charged ions combine in a ratio such that the amount of positive charge contributed by the cation equals the amount of negative charge contributed by the anion.
With that principle understood, you can answer any ion ratio question.
 Ion Cards
Ion Cards
Ion ratios can be understood using a visual tool known as
ion cards. Ion cards represent cations and anions by cards having notches and tabs on their ends. Positive ions have one or more notches. Negative ions have one or more tabs. The number of notches and tabs is equal to the amount of positive or negative charge on the ion. The notches and tabs represent charges that must be balanced to form neutral ionic compounds.
With ion cards, ionic compounds form by fitting the tabs of the anion into the notches of the cation. The cards must be put together with a ratio of cation to anion such that there are no open notches or unused tabs on any cards. Some examples are shown below. Once the compound is formed with no open notches or unused tabs, the ion ratio can be determined by counting the cation and anion cards. Examples are shown below.

Formula Units vs. Molecules
We normally imagine samples of pure compounds as consisting of molecules. A water (H
2O) sample will consist of H
2O molecules. A carbon dioxide (CO
2) sample will consist of CO
2 molecules. And a sucrose (C
12H
22O
11) sample will consist of C
12H
22O
11 molecules. These are all a type of compound known as
a molecular compound. Molecular compounds are formed from two or more non-metals.
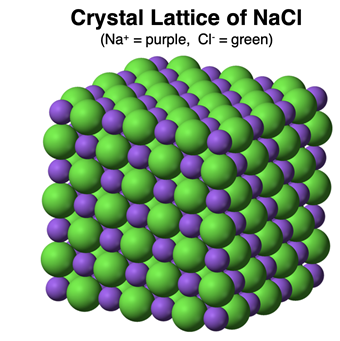
A sample of an ionic compound does not contain any molecules. That is to say, the cation and anion exists as separate ions that arranged to form a
crystal lattice. A sodium chloride (NaCl) sample does not consist of NaCl molecules. It consists of Na
+ and Cl
- ions forming an orderly and regular pattern within the lattice. Each Na
+ ion has six neighboring Cl
- ions. And each Cl
- ion has six neighboring Na
+ ions. There are no molecules.
Instead of thinking in terms of molecules, we think in terms of formula units. And that’s where the ion ratio becomes important. A
formula unit is the lowest whole number ratio of ions represented within a sample of an ionic compound. The formula unit for sodium chloride would be NaCl since the sodium ion and chloride ion are present in a 1:1 ratio. The formula unit for aluminum fluoride is AlF
3 since the aluminum ion and the fluoride ion are present with a 1:3 ratio. And the formula unit for aluminum oxide is Al
2O
3 since the aluminum ion and the oxide ion are present with a 2:3 ratio. The subscripts in the formula units represent the number of ions present in the ion ratio.
We will learn more about formula units and chemical formulas for ionic compounds on
the next page of Lesson 1.
Before You Leave
- Download our Study Card on Ion Ratios. Save it to a safe location and use it as a review tool. (Coming Soon)
- The Check Your Understanding section below include questions and problems with answers and explanations and solutions. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. The elements listed below form ionic compounds. Use their location in the
periodic table to determine
- the ion symbol with the charge, and
- the ion ratio.
The
atomic number – Z – is stated to help you more quickly locate the elements on the periodic table.
- Barium (Z=56) and iodide (Z=54)
- Magnesium (Z=12) and sulfide (Z=16)
- Potassium (Z=19) and sulfide (Z=16)
- Magnesium (Z=12) and nitride (Z=7)
- Iron(III) (Z=26) and selenide (Z=34)
- Copper(I) (Z=29) and sulfide (Z=16)
- Titanium(IV) (Z=22) and oxide (Z=8)
- Nickel(II) (Z=28) and phosphide (Z=15)
- Calcium (Z=20) and nitrate (see Polyatomic Ion list)
- Aluminum (Z=13) and acetate (see Polyatomic Ion list)
- Copper(II) (Z=29) and sulfate (see Polyatomic Ion list)
- Calcium (Z=20) and phosphate (see Polyatomic Ion list)
2. Ken Fused and Anna Litical are discussing the ion ratio of an ionic compound formed from barium ions (Ba
2+) and oxide ions (O
2-). Ken is convinced that the ion ratio is 2:2. Anna Litical knows better. Explain what Anna could say to help clear up Ken’s confusion.
3. The ion ratio for several ionic compounds made from a transition metal and an anion are given. Identify the name of the transition metal; be sure to include the Roman numeral.
- Fe to Br ion ratio is 1:2
- Cu to S ion ratio is 1:1
- Au to P ion ratio is 1:1
- Cr to S ion ratio is 2:3