Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Ions
Part a: Metals, Nonmetals, and Ions
Part 3a: Metals, Nonmetals, and Ions
Part 3b: Transition Metal Ions
Part 3c: Polyatomic Ions
Know Your Elements
As
discussed in Lesson 2, the periodic table consists of 118 elements organized into 18
groups (columns) and seven
periods (rows). The groups are numbered 1-18. Elements in groups 1-2 and 13-18 are known as
main group elements or
representative elements. There are four periods of 10 elements each in groups 3-12 that are known as
transition metal elements. There are an additional two rows of 14 elements each - known as
lanthanides and
actinides - that are typically displayed separate from the 18 columns.
In
Chapter 2, we emphasized that elements can be identified by their unique
chemical and physical properties. Based on those properties, we can divide the 118 elements into two broad groups – metals and nonmetals. A stair-step line can be drawn on the chart that divides the elements into those that are metals and those that are nonmetals. There are 6-8 elements (depending on the source) that share the properties of both metals and nonmetals that lie along the line. These elements are referred to as metalloids. Their symbols are shown below in red. Polonium (Po) and astatine (At) are the two elements for which there is the most disagreement regarding their classification as metalloids. Their symbols are shown below in grey.
As Mendeleev first noted, main group elements in the same group share the same chemical properties. The manner in which they bond to other elements to form compounds is identical. We often refer to such elements as families. Just as members of human families inherit the same physical traits, members of an elemental family inherit the same chemical traits. Four of the families have names – alkali metals (group 1), alkaline earth metals (group 2), halogens (group 17), and noble gases (group 18). It would be wise to quickly become familiar with the names of these four groups.
The Noble Gas Family

Group 18 is the noble gas family. This family consists of very non-reactive elements. We refer to them as
inert gases. Inert means
inactive or
nonreactive. Noble gases are stable as elements and seldom form compounds by reacting with other elements. It is this tendency to not interact with other elements that gained them the family name - noble gases. The general contentedness of these elements is attributed to their electronic stability. Chemists understand that particles (atoms or ions) that have 2, 10, 18, 36, 54, and 86 electrons are electronically stable.
Ions
Throughout this
Tutorial, we have referred to atoms as being electrically neutral. The number of protons (positive charges) is equal to the number of electrons (negative charges) in an atom. Atoms have a balance of the two types of charge. This is why they are electrically neutral.

Atoms of elements commonly form ions. An
ion can be thought of as an atom or molecule with a net electric charge. A neutral atom can become an ion by gaining or losing an electron. The balance of electric charge is disrupted. There are unequal numbers of electrons and protons. The particle is charged; it is an ion.
When a neutral atom gains one or more electrons, the number of electrons (negative charges) is greater than the number of protons (positive charges). The ion that is formed is a negative ion. We refer to the negatively charged ion as an
anion. When a neutral atom loses one or more electrons, the number of electrons (negative charges) is less than the number of protons (positive charges). The ion that is formed is a positive ion. We refer to the positively charged ion as a
cation.
Ion Formation
Main group elements (groups 1-2 and 13-18) exhibit very predictable patterns of ion formation. The patterns make sense in light of the discussion of noble gases above. Noble gases are electronically stable. The arrangement or configuration of electrons in noble gases is what makes them stable. Main group elements gain or lose electrons to achieve a stable noble gas configuration of electrons. Metals will lose electrons to become like a noble gas; they become positively charged cations. Nonmetals will gain electrons to become like a noble gas; they become negatively charged anions. Here are the most predictable patterns.
- Elements in the alkali metal family (group 1) lose one electron and become ions with a charge of +1.
- Elements in the alkaline earth metal family (group 2) lose 2 electrons and become ions with a charge of +2.
- Metals in group 13 (aluminum, gallium, indium, etc.) lose 3 electrons and become ions with a charge of +4.
- Metals in group 14 (tin, lead, etc.) tend to lose 4 electrons and become 4+ ions. (Tin and lead are a bit unusual in that they can form both 4+ ions and 2+ ions.)
- Nonmetals in the halogen family (group 17) gain one electron and become and become ions with a charge of -1.
- Nonmetals in group 16 (oxygen, sulfur, and selenium) gain two electrons and become ions with a charge of -2.
- Nonmetals in group 15 (nitrogen and phosphorus) gain three electrons and become ions with a charge of -3.
- The only nonmetal in group 14 (carbon) gains four electrons and becomes an ion with a charge of +4.
Ion Names, Symbols, and Ion Formation Equations

As you are likely learning, Chemistry involves a symbolic language. We represent systems using
symbols. And we represent the changes that occur within a system by
equations that include symbols. Additionally, chemistry involves a good deal of
nomenclature - a collection of rules for how to name parts of a system. Success in Chemistry will be dependent upon the attention that you give to symbols, equations, and nomenclature.
We represent ions symbolically by writing the elemental symbol for the ion following by a superscript that indicates the charge. Examples for groups 1, 2, 3, 15, 16, and 17 are shown below. The name of a monatomic cation is simply the element name followed by the word ion. A monatomic anion is a bit trickier. The trick involves removing the ending from the element name and replacing it with the -ide suffix followed by the word ion. Oxygen becomes oxide and nitrogen becomes nitride. Examples are shown below.
(
NOTE: monatomic ions are ions that include a single atom. We will discuss polyatomic ions later.)
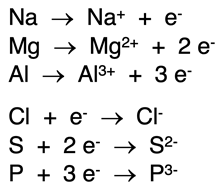
Changes occurring within a system are represented by equations. Equations includes a
yields symbol – an arrow pointing from left to right (→). The initial state of the system is displayed on the left side of the yields symbol. The final state of the system is displayed on the right side of the yields symbol. You can interpret a yields symbol as
changes into. The equations at the right represent ion formation from a neutral atom. For a gain of electrons, the electrons are shown on the left side of the yields symbol. For a loss of electrons, they are shown on the right side of the yields symbol. Notice the numbers in front of the electron symbol (known as coefficients) indicate how many electrons were gained or lost.
Isotope Symbols and Electron Cloud Diagrams
The diagrams below are sometimes referred to as
electron cloud diagrams. They represent the subatomic particles present in an atom or ion. The electrons are located outside the nucleus in an electron cloud. (This will be discussed in detail in Chapter 5.) Observe the connection between the electron cloud diagrams and the isotope symbol. Unlike neutral atoms, the isotope symbol for an ion will show a charge. The charge of the ion is written to the right of the symbol as a superscript. Examples are shown below for the beryllium ion, the fluoride ion, and the aluminum ion.
Before You Leave
- Download our Study Card on Ions. Save it to a safe location and use it as a review tool.
- The Check Your Understanding section below include questions and problems with answers and explanations and solutions. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Of the six listed elements, identify the three pairs that would most likely have similar chemical properties:
| Chlorine (Cl) |
Sodium (Na) |
Strontium (Sr) |
| Iodine (I) |
Calcium (Ca) |
Potassium (K) |
2. Atoms are neutral and not charged because …
- They have neutrons.
- They have more neutrons than either protons or electrons.
- They have equal numbers of neutrons and protons.
- They have equal numbers of electrons and protons.
3. An anion is a __________ ion. A cation is a __________ ion.
- positive, negative
- negative, positive
4. A neutral atom becomes positively-charged by _____________.
- gaining protons
- gaining electrons
- losing protons
- losing electrons
5. A neutral atom becomes negatively-charged by _____________.
- gaining protons
- gaining electrons
- losing protons
- losing electrons
6. Metals ________ electrons and become _____________ ions.
- gain, positive
- gain, negative
- lose, positive
- lose, negative
7. Nonmetals ________ electrons and become _____________ ions.
- gain, positive
- gain, negative
- lose, positive
- lose, negative
8. Complete these sentences:
An atom of aluminum (Al) becomes a stable ion by ____________ (gaining, losing) ______ (a number) electrons and assuming an electron configuration that resembles that of the element ___________ (name or symbol).
An atom of phosphorus (P) becomes a stable ion by ____________ (gaining, losing) ______ (a number) electrons and assuming an electron configuration that resembles that of the element ___________ (name or symbol).
9. Write the equation for the formation of the …
- strontium ion from the Sr atom
- cesium ion from the Cs atom
- selenide ion from the Se atom
- nitride ion from the N atom
10. For each of the given electron cloud diagrams, write the name of the ion and its isotope symbol.