Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Ionic Compounds
Part c: Binary Ionic Compounds
Part 1a: Properties of Ionic Compounds
Part 1b: Combining Ions to Form Ionic Compounds
Part 1c: Binary Ionic Compounds
Part 1d: Compounds Containing Polyatomic Ions
What is a Binary Ionic Compound?
A binary ionic compound is an ionic compound that contains only two elements. One of the elements is a metal and serves as the cation (+). The other element is a nonmetal and serves as the anion (-). Here are a few examples of binary ionic compounds, written in formula form.
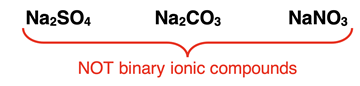
Binary ionic compounds typically do not contain polyatomic ions. Most polyatomic ions consist of two or more elements. Once the ion forms a compound with an ion of opposite charge, the number of elements exceeds two. By definition, it is not a binary ionic compound. As an example, if sulfate (SO
42-) or carbonate (CO
32-) or nitrate (NO
3-), all polyatomic ions, forms an ionic compound with a cation like Na
+, the result is an ionic compound with three elements. This is shown at the right. There are a small number of exceptions of polyatomic ions (contain two or more atoms) that contain only one element. The peroxide ion (O
22-) and the azide ion (N
3-) are examples. When these polyatomic ions combine with another element to form ionic compounds, the result is a binary ionic compound.
Two Types of Binary Ionic Compounds
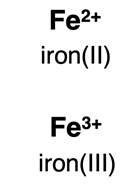
We distinguish between two types of binary ionic compounds – Type I and Type II. In
Type I binary ionic compounds, the two elements are main group elements. The charge of the ions can be determined by their location within the periodic table. Ions of main group elements were discussed thoroughly in Chapter 3. (Review
ions of main group elements.)
Type II binary ionic compounds include a transition metal ion and a nonmetal ion. Most transition metal ions are
multivalent; that is, they form ions that have more than one charge. For instance, iron can be Fe
2+ or Fe
3+. When naming these two ions of iron, we distinguish between them by using a Roman numeral in parenthesis – iron(II) and iron(III). Transition metal ions were discussed thoroughly in Chapter 3. (Review
ions of transition metal elements.)
Of course there are always exceptions. It’s the exceptions to the rule that makes it difficult in Chemistry to
keep all things straight. One notable exception is that lead (Pb) and tin (Sn) are two main group elements that are also multivalent. While they form the expected 4+ ion, they also form a 2+ ion. As such, Roman numerals are used to distinguish between the two ions of these elements.
The reason we distinguish between Type 1 and Type II binary ionic compounds is because the methods of naming them and writing chemical formulas for them will vary slightly.
Nomenclature

Nomenclature is a collection of rules for naming the parts of a system. All fields of science and non-science typically include such rules. In Chemistry, the rules are essential as they allow chemists to communicate clearly about a vast array of chemicals. Slight alterations in the naming of a chemical can be the difference between life and death. Carbon
dioxide is a component in the air exhaled by the living. Carbon
monoxide is a poisonous gas. The prefixes di- and mon- on the second word are the difference between life and death.

The goal of nomenclature is to get the world to speak the same language such that the words we use to refer to chemical substances leaves no ambiguity. The names of binary ionic compounds always include two separate words. The first word is the name of the metal ion. The second word is the name of the nonmetal ion. Names of ions were discussed in
Lesson 3 of Chapter 3.
For Type II binary ionic compounds: An ion of a transition metal element (and also of lead and tin) can have multiple charges. A roman numeral enclosed in parentheses must follow the name of the metal so as to leave no ambiguity regarding what ion is in the compound.
From Formula to Name
A common task for Chemistry students is to identify the name of an ionic compound if given its formula. Identifying CaCl
2 as calcium chloride and Fe
2O
3 as iron(III) oxide is a skill used throughout the course. To do so, you must first identify the ions. The metal ion comes first in the formula. Use a periodic table to determine the name associated with the symbol. You must determine the charge for transition metals, lead, and tin. The charge can be determined by using what we learned about ion ratios on the previous page. The nonmetal ion comes second. Remember the -ide ending for main group nonmetals. Once you have identified the ions, use the two steps (above) to write the name of the ionic compound. Here are some examples.
The Roman numeral is included for transition metals, lead, and tin since they can have more than one ion charge. This is the case in rows
e through
n. Give attention to the charge on the anion and the subscripts in the formula to determine the ion charge. Be guided by the principle that the net charge of an ionic compound is 0. The sum of all negative charge from the anion balances the sum of all positive charge from the cation. Here’s how the principle is applied for row k through n.
 Formula Writing
Formula Writing
Chemistry students need to learn how to write the formula of an ionic compound if given the name of the ionic compound. The name consists of two words. The first word is the cation (+ ion). The second word is the anion (- ion). Use the periodic table to determine the symbol and the charge of the cation and the anion. (Review
Lesson 3 of Chapter 3 if necessary.) If the cation is a transition metal, lead, or tin, then the charge will be indicated in the name with a Roman numeral inside parenthesis. Write down the symbols with the charge for the two ions. Then determine the
ion ratio knowing that the ionic compound is overall neutral. (Review
Lesson 1b of this chapter or use our
Study Card.) The
ion ratio can be used to determine the subscripts in the formula. If a subscript is 1, then it is usually not written. The table provides several examples.
Criss-Cross Method for Formula Writing
When it comes to formula writing, many Chemistry students prefer the so-called
crisscross method. The crisscross method bypasses the task of determining the ion ratio. The method goes like this:
- Determine the symbol of the cation; include the charge.
- Determine the symbol of the anion; include the charge.
- Write the formula from the symbols. The charge on the cation is the subscript on the anion symbol. The charge on the anion is the subscript on the cation symbol.
- If needed, reduce the subscripts to the lowest whole numbers. For instance, Ca2O2 can be reduced to CaO.
The examples below show the crisscross method being used to write formulae from names.
Before You Leave
- Download our Study Card on Binary Ionic Compounds. Save it to a safe location and use it as a review tool.
- It was discussed on a different page, but if you missed it, then you might want to visit our Ion Ratio page or even download the Study Card on Ion Ratios.
- Try our Concept Builder titled Names to Formula 1. All three activities in the Concept Builder would provide great interactive practice with identifying the formulas for Type I and Type II binary ionic compounds.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready. Use our
Periodic Table if necessary.
1. Explain when a Roman numeral inside of parenthesis should be used when naming a compound.
2. What’s Wrong with This?
A teacher is grading a quiz on nomenclature. She sees the following formulas. Identify what is wrong with each formula.
- Calcium chloride → Ca2Cl
- Barium oxide → Ba2O2
- Aluminum chloride → Al1Cl3
- Tin(IV) oxide → Sn2O
- Sodium selenide → Na-2Se
- Chromium(V) nitride → N5Cr3
3. And What’s Wrong with This?
A teacher is grading a quiz on nomenclature. He sees the following names of ionic compounds. Identify what is wrong with each name.
- Al2O3 → aluminum(III) oxide
- PbI2 → lead iodide
- BaSe → selenium baride
- TiO2 → titanium(II) oxide
4. Determine the names associated with the following formulas.
- ZrI4 →
- AlBr3 →
- CuS →
- Ti3N4 →
- Rb2O →
- MnO2 →
- NiSe →
- Ag2S →
- GaCl3 →
5. Determine the formulas associated with the following names.
- strontium fluoride →
- copper(II) selenide →
- calcium sulfide →
- vandium(V) oxide →
- chromium(V) nitride →
- manganese(IV) fluoride →
- platinum(IV) oxide →
- gold(I) chloride →