Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: The Periodic Table Revisited
Part c: Isotopes and Isotope Symbols
Part 2a: Mendeleev and the Periodic Law
Part 2b: Today's Periodic Table
Part 2c: Isotopes and Isotope Symbols
Atomic Number
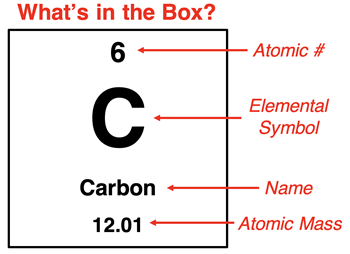
Atoms contain protons, neutrons, and electrons. The numbers of each varies with the element. The one identifying feature of any element is the number of protons in the nucleus. The number of protons is referred to as the
atomic number. The atomic number is the integer that is written in every cell of a periodic table. It provides a measure of the amount of positive charge in the nucleus of an atom. An atom is an electrically neutral particle; the number of electrons must equal the number of protons.
The element name, elemental symbol, and the number of protons go together. Every atom of carbon (C) contains six protons and has an atomic number of 6. Every atom of nitrogen (N) contains seven protons and has an atomic number of 7. And every atom of oxygen (O) contains eight protons and has an atomic number of 8. You will never find an atom of carbon with 7 protons since all carbon atoms have 6 protons. If you found an atom with seven protons, it would be called a nitrogen atom.
Mass Number

The mass number indicates the relative mass of a nucleus. The
mass number is equal to the number of protons plus neutrons in a nucleus. A boron atom with five protons and four neutrons has an atomic number of 5 and a mass number of 9. A phosphorus atom with 15 protons and 16 neutrons has an atomic number of 15 and a mass number of 31. And a chlorine atom with 17 protons and 20 neutrons has an atomic number of 17 and a mass number of 37.
Let’s make sure you got it! Use the ideas above and a periodic table to determine the missing cell values in the following table. Tap the buttons to see how you did.
Isotopes
Every atom of a particular element always has the same number of protons. But the number of neutrons for a particular element can vary from atom to atom. For example, all hydrogen atoms have 1 proton. But there are three different forms of hydrogen that have a differing number of neutrons. The most common form is called
protium; it has no neutrons.
Deuterium has one neutron. And
tritium has two neutrons.
Forms of the same element that have different numbers of neutrons are referred to as
isotopes. The three forms of hydrogen are referred to as the isotopes of hydrogen. Nearly every element has two or more isotopes. To distinguish between the individual isotopes of an element, it is customary to append the mass number to the end of the name. For instance, protium is referred to as hydrogen-1. Deuterium is hydrogen-2. Tritium is hydrogen-3.
The name, composition, and relative abundance in nature of the three isotopes of carbon and of chlorine are shown below. The relative abundance of an isotope is one of the factors that affects the atomic mass of an element. We will have much more to say about this in
Chapter 7 of this
Chemistry Tutorial.
Isotope Symbols
An isotope is often represented by an isotope symbol. An isotope symbol consists of four parts:
- An elemental symbol
- An atomic number
- A mass number
- The overall charge of the particle
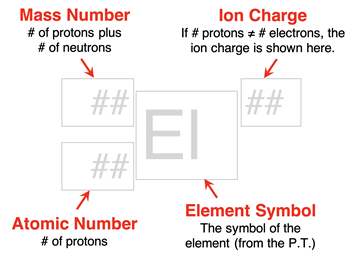
The atomic number and elemental symbol will always match. If you know one, then you can find the other using a periodic table. The atomic number is written as a
subscript to the left of the elemental symbol. The mass number is written as a
superscript to the left of the elemental symbol. The overall charge of the particle (# of protons - # of electrons) is written as a
superscript to the right of the elemental symbol. An atom has an overall charge of 0. When the overall charge is zero, the charge is generally not written. Ions (to be discussed in
Lesson 3) have an overall charge; a superscript is included for ions.
Here are isotope symbols for atoms of four different isotopes.
Putting It All Together
Often times the challenge for students is to understand and apply all the relationships. The following bullet points summarize the relationships.
- The elemental symbol and the atomic number go together; they can be found on the periodic table.
- The atomic number equals the number of protons.
- The mass number, number of protons, and number of neutrons are related by
Mass number = # of protons + # of neutrons
- The name of an isotope is written in the form: element name + hyphen + mass number.
- Atoms are neutral. Therefore, # of protons = # of electrons.
Before You Leave
- Download our Study Card on Isotopes. Save it to a safe location and use it as a review tool.
- Try our Concept Builder titled Subatomic Particles. The Apprentice Difficulty Level will be a great follow-up to this page.
- Try our Concept Builder titled Isotopes. The first two activities will be great practice.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Can the number of electrons in an atom ever be equal to the number of neutrons?
2. Can the number of electrons in an atom ever be different than the number of protons?
3. Explain how you can determine the number of neutrons in the iron-56 isotope.
4. Are oxygen-19 and fluorine-19 isotopes?
5. How would you name the isotope that has 35 protons and 45 neutrons?
6. Use the information from the
Putting It All Together section to complete the following table: