Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Quantitative Analysis of Compounds
Part a: Molar Mass of Compounds
Part a: Molar Mass of Compounds
Part b:
Grams-Moles-Particles Relationship
Part c:
Percent Composition
Part d:
Empirical and Molecular Formulae
 Molar Mass Concept
Molar Mass Concept
The concept of molar mass was introduced in Lesson 1c of this chapter of the Chemistry Tutorial. The molar mass of a substance is the mass in grams of 1 mole of that substance. For elements, the molar mass is indicated on the periodic table for each element. It is the non-integer value listed in each cell. The units are grams per 1 mole. This is abbreviated as g/mol. For helium, the molar mass is 4.00 g/mol.
Molar Mass of Compounds
Compounds were first introduced in Lesson 1b of Chapter 2. A compound is a type of substance composed of two or more  elements. Hydrogen and oxygen are not compounds. They are elements. You know that they are elements because they are listed on the Periodic Table of Elements. When hydrogen and oxygen react with one another, they form a new type of substance known as dihydrogen monoxide. You can detect two elements in the name. That’s a compound. Less formally, we refer to dihydrogen monoxide as water (H2O). Water is a compound because it is composed of two or more elements. Hydrogen peroxide (H2O2) is another compound composed of the elements hydrogen and oxygen.
elements. Hydrogen and oxygen are not compounds. They are elements. You know that they are elements because they are listed on the Periodic Table of Elements. When hydrogen and oxygen react with one another, they form a new type of substance known as dihydrogen monoxide. You can detect two elements in the name. That’s a compound. Less formally, we refer to dihydrogen monoxide as water (H2O). Water is a compound because it is composed of two or more elements. Hydrogen peroxide (H2O2) is another compound composed of the elements hydrogen and oxygen.
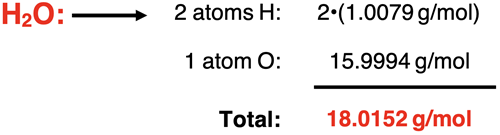
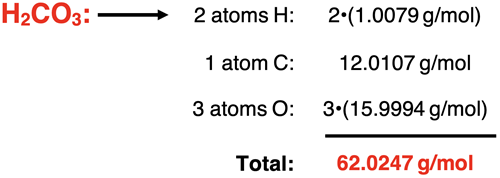
To determine the molar mass of a compound, determine the number of atoms of each element found in the compound. Then add the molar masses of each element; be sure to account for the number of atoms of each element present in the compound. Here are four examples for compounds that contain hydrogen, carbon, and oxygen.

Example 1: carbon monoxide (CO)

Example 2: carbon dioxide (CO2)

Example 3: dihydrogen monoxide (H2O)
Example 4: carbonic acid (H2CO3)
Interpreting a Formula
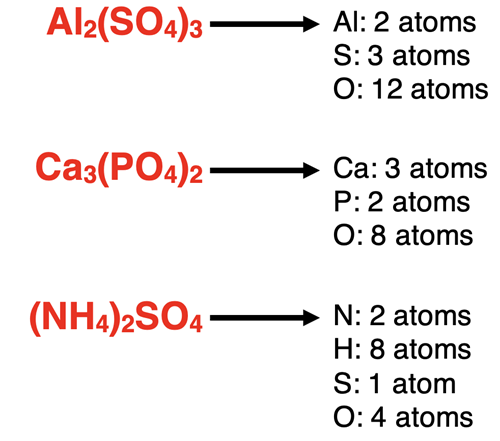
A common challenge involves the task of interpreting a formula to determine the number of atoms of each element. This is most challenging when the formula contains parenthesis. When you see parenthesis with a subscript following them, it indicates that there are multiples of everything inside the parenthesis. For instance, the formula for calcium nitrate is Ca(NO3)2. The subscripted 2 following the parenthesis indicates that there are two of everything inside the parenthesis.

So, Ca(NO3)2 consists of 1 Ca atom, 2 N atoms, and 6 O atoms.
Here are three more examples. Note how the subscripts of the elements inside the parenthesis are multiplied by the subscript outside the parenthesis.
More Practice
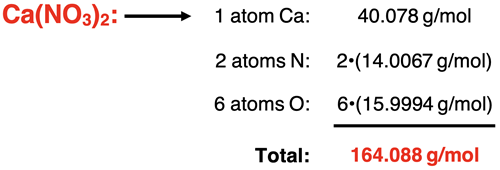
Here are three more examples of molar mass calculations.
Example 5: calcium nitrate - Ca(NO3)2
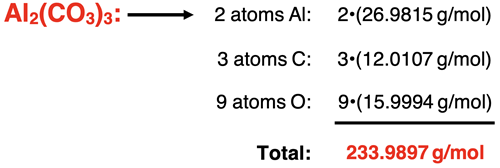
Example 6: aluminum carbonate – Al2(CO3)3
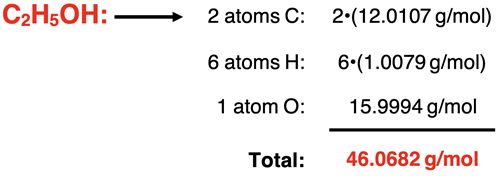
Example 7: ethanol – C2H5OH
Chemistry is not a great spectator sport. You typically don’t learn the skills by watching another person perform. Every chemistry student needs to practice. Take advantage of our Check Your Understanding section or the resources mentioned in the Before You Leave section.
How Many Digits are Necessary?
The question often arises as to how many digits or decimal places should one use when performing molar mass calculations. If you are taking Chemistry for a class, then the best answer is that you should use as many decimal places as your instructor requires. The generally accepted answer pertains to significant digits as was discussed in Chapter 1 of this Chemistry Tutorial. As we will see in the next part of Lesson 2, the calculation of molar mass is usually one step in a multi-step problem. The molar mass value is typically used in subsequent calculation steps of the problem. As such, attention should be given to the number of significant digits of other quantities in the calculations. It is important that you have at least as many significant digits for the molar mass as you have on the other quantities that will be multiplied or divided by it. For instance, if you will be dividing a number with four significant digits by your molar mass value, then you should have at least four significant digits in your molar mass calculation.
Before You Leave
- Download our Study Card on Molar Mass. Save it to a safe location and use it as a review tool.
- Our Calculator Pad section has two sets of Practice Problems on this topic. Your answers are evaluated and feedback is provided; you have infinite opportunities to correct your mistakes. Try …
- Try our Concept Builder titled Molar Mass. It begins with an atom counting exercise and ends with a field trip to Planet Mayedup. It's out of this world. Each question provides a great structure that matches the structure provided in this Tutorial. Give it a try.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Determine the molar mass of the following compounds.
- H2C2O4
- Na2SO4
- Al(C2H3O2)3
2. Write the chemical formula and determine the molar mass of the following molecular compounds. If necessary, review
the rules for formula writing for molecular compounds.
- diphosphorus pentoxide
- dinitrogen trioxide
3. Write the chemical formula and determine the molar mass of the following ionic compounds. If necessary, review
the rules for formula writing for ionic compounds.
- iron(II) nitrate
- calcium sulfate