Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Molecular Compounds
Part b: Names and Formulas of Molecular Compounds
Part 2a: Properties of Molecular Compounds
Part 2b: Names and Formulas of Molecular Compounds
Binary Molecular Compounds
A
binary molecular compound consists of atoms of two nonmetal elements. Examples include H
2O, CH
4, NH
3, CO
2, PCl
3, etc. When a main group metal and nonmetal form an ionic compound, the formula of that compound is predictable. Calcium and chlorine always form CaCl
2 and aluminum and oxygen always form Al
2O
3. This is not the case for molecular compounds. Carbon and oxygen can form CO or CO
2. Sulfur and oxygen can form SO
2 or SO
3.
Formulas to Names
The name of a molecular compound must communicate which elements and the number of atoms of each that are in the compound. This communication must be done such that the formula and identify of the compound is clearly known without any ambiguity.
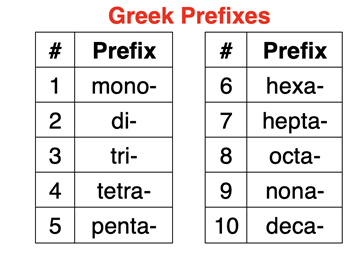
Every binary molecular compound consists of two words. The first word is the name of the first element listed in the formula. The second name is for the element listed in the formula. The ending of the second element is removed and replaced with the -ide suffix. Each name is preceded by Greek prefixes to indicate the number of atoms. See table at right. The prefix mono- is never used in front of the first name; it is however used in front of the second name when appropriate. If you don’t see a prefix in front of the first element, then assume there is only one of that atom. If a Greek prefix ending in “a” is followed by an element name starts with “o”, then the a is removed. Instead of tetraoxide, the “a” is dropped and tetroxide is used.
Here are several examples of names being derived from their formulas.
There is additional practice in the
Check Your Understanding section.
Names to Formulas
If you understand how to name a molecular compound, the determination of the formula from the name is a straightforward task. But be aware that subscripts are never reduced to the lowest whole number ratio. Tetrasulfur decoxide is S
4O
10 and is not reduced to S
2O
5. The name tetrasulfur decoxide refers to a molecule that contains 4 atoms of sulfur and 10 atoms of oxygen. This is quite different than ionic compounds in which the subscripts are reduced where applicable. As discussed in Lesson 1, ionic compounds are not composed of molecules and the formula states the simplest, whole number ratio of ions in the crystal lattice.
Here are several examples of formulas being derived from names.
There is additional practice in the
Check Your Understanding section.
Before You Leave
- Download our Study Card on Binary Molecular Compounds (Names and Formulae). Save it to a safe location and use it as a review tool.
- Try our Concept Builder titled Names to Formula 2. The first activity (Apprentice Level) would provide great practice with identifying the formulae for binary molecular compounds.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Indicate the name that is consistent with the following molecular formulae.
- SiO2 →
- N2O →
- NCl3 →
- P2O5 →
- SCl4 →
<
- XeF6 →
- IF7 →
- N2O4 →
2. Indicate the formula that is consistent with the following names.
- dihydrogen monoxide →
- sulfur tetrachloride →
- tetraphosphorus hexafluoride →
- xenon tetrafluoride →
- dichlorine heptoxide →
- carbon tetrachloride →