Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 4: Periodic Trends
Part c: Ionization Energy
Part 4a:
The Periodic Law Revisited
Part 4b:
Atomic Size
Part 4c: Ionization Energy
Part 4d:
Electronegativity
What is Ionization Energy?
Ionization energy is a periodic property. When progressing from element to element in order of atomic number, there is a pattern of change that repeats itself over and over again. Ionization energy is defined as the amount of energy required to remove an electron from an isolated atom.

More than one electron can be removed from an atom. Each electron that is removed requires its own unique  amount of energy. A distinction is made between the first ionization energy (energy to remove the first electron), the second ionization energy (energy to remove a second electron), the third ionization energy, etc. In this lesson, we will discuss first ionization energies and simply refer to it as ionization energy. The unit used to express ionization energy is the electron-volt, abbreviated eV.
amount of energy. A distinction is made between the first ionization energy (energy to remove the first electron), the second ionization energy (energy to remove a second electron), the third ionization energy, etc. In this lesson, we will discuss first ionization energies and simply refer to it as ionization energy. The unit used to express ionization energy is the electron-volt, abbreviated eV.
There is an electrical force of attraction between a positively charged nucleus (protons) and every electron in an atom. Energy is always required to overcome this attraction and remove the electron. The amount of energy required is primarily dependent upon how far the electron is from the nucleus and the amount of charge on the nucleus. The force of electrical attraction decreases with an increasing distance from the nucleus. And the force of electrical attraction increases with increasing nuclear charge. Sometimes these two factors compete against each other.
A Look at the Data
The scatter plot below displays ionization energy data for the main group elements in periods 1 through 6. The atomic number is plotted along the x-axis. The gaps (areas of no data points) between atomic numbers 20 and 31, between atomic numbers 38 and 49, and between atomic numbers 56 and 81 are transition metals and lanthanides. Data for these elements are not included.
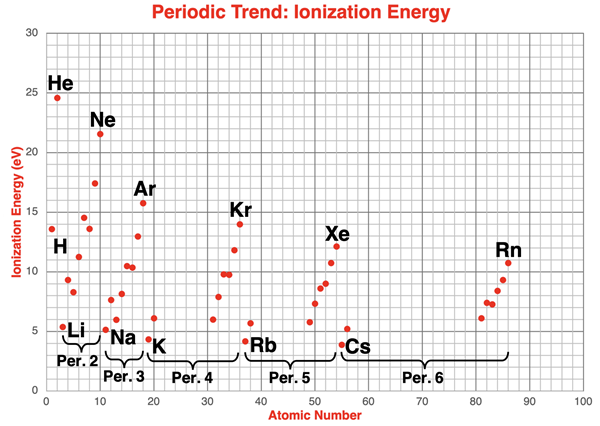
Points at the beginning (alkali metals) and the end of a period (noble gases) are labeled with their elemental symbol. The pattern of change or trend from H to He (period 1), from Li to Ne (period 2), from Na to Ar (period 3), from K to Kr (period 4), from Rb to Xe (period 5), and from Cs to Rn (period 6) is clearly evident. The pattern of an increasing ionization energy repeats itself during each of these periods.
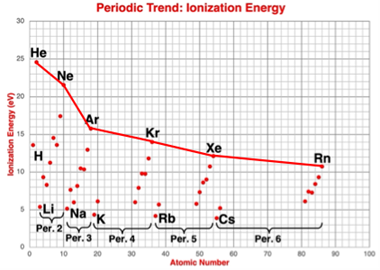
Describing the Trends
Two trends can be identified in the above graph.
- As one proceeds from left to right across a period of the Periodic Table, the ionization energy generally increases.
- As one proceeds from top to bottom down a group of the Periodic Table, the ionization energy decreases.
Some Finer Details
The first trend – the across a period (row) trend – is quite obvious from an inspection of any of the
periods displayed on the graph. There are two sets of elements in both period 2 and period 3 that deviate from the general trend and show a slight decrease in ionization energy with an increasing atomic number. In period 2, this occurs from
4Be to
5B
and from
7N to
8O. In period 3, this occurs from
12Mg to
13Al and from
15P to
16S. There are some other less obvious deviations from the general trend in the other
periods. These occur from the group 15 to the group 16 element.
The second trend – the down the group (column) trend – is not as obvious. It is most noticeable if you compare the values of ionization energy for
the noble gases. Progression from He to Ne to Ar … to Rn show that the ionization energies are constantly decreasing as you progress to elements that are lower in the noble gas family. A careful inspection of the other groups reveals the same pattern of change.
Explaining the Trends
The
modern model of the atom can be used to explain the two trends. When progressing from element to element across a
period of the periodic table, one electron and one proton are being added with each successive element. The added electrons are entering the same
valence shell. The addition of a proton has two effects that lead to higher ionization energy. First, an additional proton increases the quantity of positive charge in the nucleus. This in turn increases the force of electrical attraction with the
outer shell electrons. Second, an additional proton pulls the electron cloud inwards and decreasing the size of the
valence shell. Forces of electrical attraction increase with decreasing distance. For atoms with more protons, the valence shell electron is held more tightly to the nucleus and requires a greater amount of energy to be removed.
When progressing from atom to atom down a
group of the periodic table, the number of valence electrons remains constant. But in each successive row, the valence shell electrons are entering orbitals of a different
principal energy level (n). Orbitals with a larger n value (at the bottom of the table) are larger orbitals. The
valence shell electron that will be removed is further from the nucleus and is held less tightly by the electrical force of attraction that the nucleus exerts on it. These electrons are also at higher energy levels. As such, less energy is required to remove an electron from these larger atoms.
Electron Stability
Atoms with a full set of
valence shell orbitals are significantly more stable than those that have
vacancies in their outer shell. An
s2p6 electron configuration is as stable a configuration as can be achieved. This is evidenced by the fact that the highest ionization energies are for the noble gas elements. A stable electron can be thought of as a contented electron and it’s not going to be happy about departing from its stable state.
Before You Leave
- Practice. Try our Periodic Trends Concept Builder. It’s great practice! The second of the three activities pertains to ionization energy.
- Download our Study Card on Periodic Trends. Save it to a safe location and use it as a review tool. This Study Card covers Lesson 4b – Lesson 4d.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. For each set of three elements, use the periodic trends to rank the elements in order of increasing ionization energy:
- 3Li, 19K, 55Cs
- 4Be, 6C, 9F
- 56Ba, 82Pb, 85At
- 10Ne, 17Cl, 18Ar
- 35Br, 56Ba, 85At
- 3Li, 4Be, 19K
2. In general, in which area of the periodic table would you be more likely to find elements with the lowest ionization energies?
- Top left
- Top right
- Bottom left
- Bottom right