Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Electrons
Part b: Electrons and the Periodic Table
Part 3a:
Electron Configurations
Part 3b: Electrons and the Periodic Table
Part 3c:
Exceptions to the Rules
Patterns
We learned how to write electron configurations on the previous page of Lesson 3. Our method relied on an understanding of the order by which electrons fill the orbitals. An energy level diagram or orbital box diagram was an essential tool in the process of writing the electron configuration. On this page, we will learn a quicker and easier method to writing electron configurations. All it requires is a periodic table and a recognition of the patterns relating an element’s location on the table to the configuration of electrons within orbitals.
The Periodic Table is all about patterns. It’s development in the 19th century was motivated by the recognition of patterns in the chemical properties of the elements. The periodic law guided the process of organizing elements into periods and groups. Unknown at the time, the underlying reasons for the patterns was the electronic structure of the atoms. Now we will relate the structure of the electrons to the location of the elements on the table.
The s Block
Consider the abbreviated electron configurations of the elements in Groups 1 and 2 of the periodic table. Put on your detective cap and see if you can find the pattern.
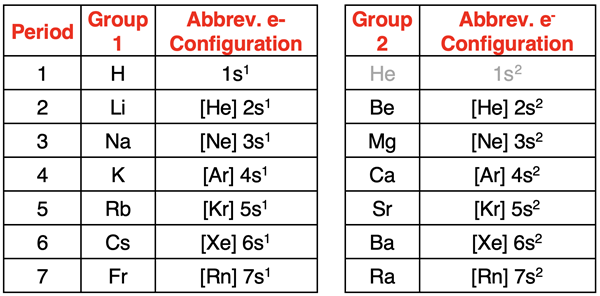
 The pattern is that all elements in Group 1 end with ns1 where n is the period number. All elements in Group 2 end with ns2 where n is the period number. For this reason, we refer to Groups 1 and 2 as the s-block of the periodic table. The last of the outer shell electrons for those elements are entering into the s orbitals. Group 1 elements will always have one valence shell electron, and Group 2 elements will always have two valence shell electrons. Understanding this pattern provide an easy method for writing abbreviated electron configurations. Note that for the purposes of this exercise, the element helium is best inserted beside hydrogen in the first row.
The pattern is that all elements in Group 1 end with ns1 where n is the period number. All elements in Group 2 end with ns2 where n is the period number. For this reason, we refer to Groups 1 and 2 as the s-block of the periodic table. The last of the outer shell electrons for those elements are entering into the s orbitals. Group 1 elements will always have one valence shell electron, and Group 2 elements will always have two valence shell electrons. Understanding this pattern provide an easy method for writing abbreviated electron configurations. Note that for the purposes of this exercise, the element helium is best inserted beside hydrogen in the first row.
The p Block
Now let’s repeat this pattern-finding exercise for elements in Groups 13 to 18. Can you see the pattern?

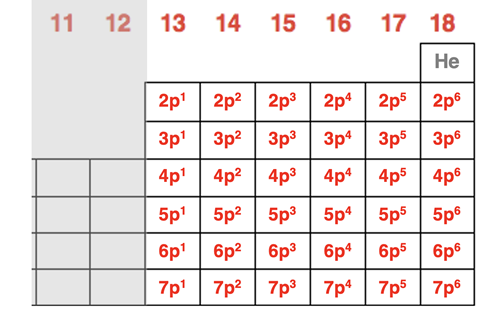
The pattern is that all elements in these six groups end with npx where n is the period number and the superscript x is a number between 1 and 6. For group 13 elements, x is 1. For group 14 elements x is 2. This pattern continues to group 18 elements where x is 6. The last of the outer shell electrons for these elements are entering into the p orbitals. For this reason, we refer to Groups 13 through 18 as the p-block of the periodic table. The p-block includes six elements. And with three p orbitals capable of holding two electrons each, the p orbitals at any principal energy level have a capacity for six electrons. You will note that the only element in period 1 is helium. For the purposes of this exercise, helium is more aptly located in the s-block next to hydrogen. This would leave no elements in the p-block for period 1. This is consistent with the fact there are no p orbitals in the n=1 principal energy level. A summary is shown below.
The d Block
Now we will inspect the transition metals - elements in Groups 3 to 12. Can you see the pattern?

(NOTE: exceptions to the theoretical predictions above will be discussed in Lesson 3c.)
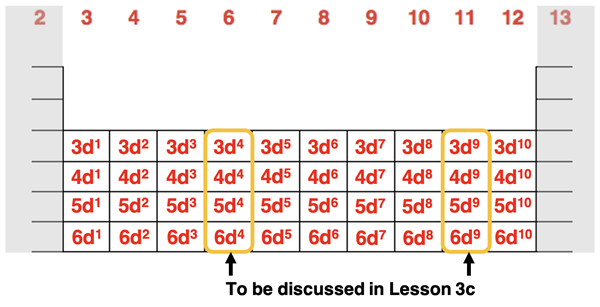
The pattern is that all elements in these 10 groups end with (n-1)dx where n is the period number and the superscript x is a number between 1 and 10. For group 3 elements, x is 1. For group 4 elements x is 2. For group 5 elements, x is 3. This pattern continues to group 12 elements where x is 10. The last of the electrons for these elements are entering into the d orbitals. For this reason, we refer to Groups 3 through 12 as the d-block of the periodic table. The d-block includes 10 elements. Since the five d orbitals can hold up to two electrons each, the d orbitals at any principal energy level have a capacity for 10 electrons.
 You might recall in our discussion of energy levels that we mentioned that the 3d orbitals are lower in energy than the 4s orbitals. As such, the 4s orbitals are filled before the 3d orbitals. It makes sense that the d block begins in period 4 after the s block of elements. Even though transition metal elements in period 4 are filling the 3d orbitals, the location of these elements on the periodic table is in the same row as the 4s and 4p elements.
You might recall in our discussion of energy levels that we mentioned that the 3d orbitals are lower in energy than the 4s orbitals. As such, the 4s orbitals are filled before the 3d orbitals. It makes sense that the d block begins in period 4 after the s block of elements. Even though transition metal elements in period 4 are filling the 3d orbitals, the location of these elements on the periodic table is in the same row as the 4s and 4p elements.
A summary is shown below.
Note that the above configurations are based on the theoretical predictions of quantum mechanics. There are a few exceptions of atoms observed behaving differently than the model predicts. The most notable exceptions involve chromium (atomic number 24) and copper (atomic number 29). We will discuss those observations in Lesson 3c and suggest some explanations for why such exceptions are observed.
The f Block
The final block of the periodic table is the so-called f-block. The f-block includes the lanthanides and the actinides. These are the 14 elements that are shown as a pull-out from the main table in periods 6 and 7. While there is some dispute among chemists as to which element begins the f-block, we will base our discussion upon the theoretical prediction of quantum mechanics. The model predicts that the 4f orbitals are lower in energy than the 5d orbitals.
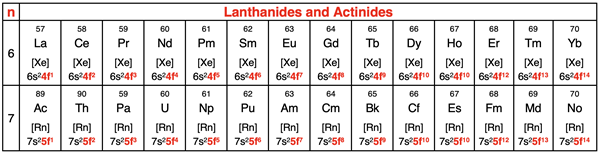
As such the element coming after barium (that’s lanthanum with an atomic number of 57) is the first f-block element. And similarly for period 7, actinium (atomic number 89) is the first f-block element. There are 14 elements in each row of lanthanides and actinides. And consistent with the model, there are seven f orbitals that can hold a maximum of 2 electrons each. The f orbitals at any principal energy level have a capacity for 14 electrons. This is a match to the 14 elements in each row of the f-block.
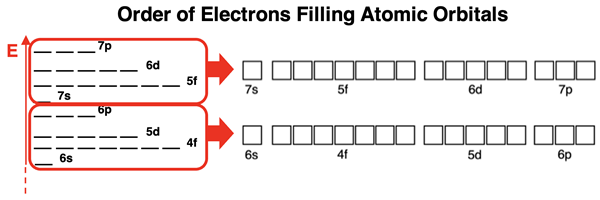
The electron configuration for an f-block element ends with (n-2)fx where n is the period number, and the superscript x is a number between 1 and 14. The value of x depends on how many elements into the f-block that an element is located. The value of x is 2 for the second element in the row and 10 for the tenth element in the row.
Putting It All Together
The graphic below summarizes the four orbital blocks of the periodic table.
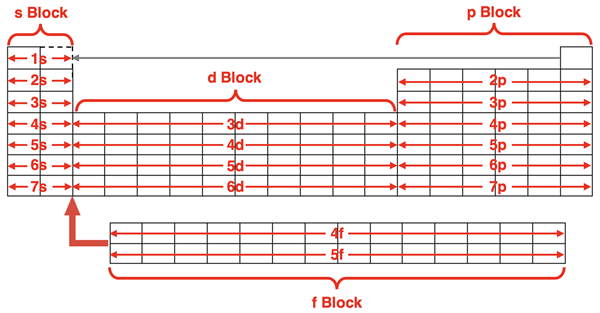
Using this understanding will help you make quick work of writing abbreviated electron configurations for an element. Here’s a brief procedure.
- Locate the element on the periodic table.
- Identify noble gas at the end of the row above the element. Enclose the noble gas symbol in brackets.
- Drop back to the next row and begin writing the remainder of the electron configuration beginning with the s-block.
Here’s three examples that use the color-coded periodic table below:
Example 1: Write the abbreviated electron configuration for phosphorus (
15P), in green.
Answer: [Ne] 3s2 3p3
Discussion: P is located in the 3rd row. Use the noble gas at the end of the 2nd row (Ne); enclose its symbol in brackets. Then starting at element #11 (sodium), include the two electrons in the 3s orbitals (3s2). Then count three elements into the 3p block to phosphorus. Use 3p3 to describe these three electrons.
Example 2: Write the abbreviated electron configuration for tin (
50Sn), in yellow.
Answer: [Kr] 5s2 4d10 5p2
Discussion: Sn is located in the 5th row. Use the noble gas at the end of the 4th row (Kr); enclose its symbol in brackets. Then starting at element #37 (rubidium), include the two electrons in the 5s orbitals (5s2). Include the 10 electrons (4d10) in the 4d orbitals (elements #39-48). Then count two elements into the p block to tin. Use 5p2 to describe these two electrons.
Example 3: Write the abbreviated electron configuration for platinum (
78Pt), in purple.
Answer: [Xe] 6s2 4f14 5d8
Discussion: Pt is located in the 6th row. Use the noble gas at the end of the 5th row (Xe); enclose its symbol in brackets. Then starting at element #55 (cesium), include the two electrons in the 6s orbitals (6s2). Include the 14 electrons (4f14) in the 4f orbitals (elements #57-70). Then count eight elements across the transition metal block to platinum. Use 5d8 to describe these eight electrons.
Valence Shell Electrons
We discussed valence shell electrons in Lesson 3a. The valence shell electrons are the electrons in the outermost s and p orbitals of atoms. The d and f orbital electrons are not valence shell electrons. It is easy to see why if we consider the electron configuration for an element such as lead:
[Xe] 6s2 4f14 5d10 6p2
The outer shell of electrons for lead are the n=6 shell. The highest energy f orbital electrons are in the n=4 shell. The highest energy d orbital electrons are in the n=5 shell. The n=4 and n=5 shells comprise the core electrons and are not outer shell or valence shell electrons. With our new model relating the electron configuration to the periodic table, it is considerably easier to count the valence shell electrons by counting across the s and p blocks to the element.
Here’s three examples that use the color-coded periodic table below:
Example 1: Determine the number of valence shell electrons in selenium (
34Se, in green).
Answer: 6
Discussion: Selenium is in group 16. Its configuration is [Ar] 4s2 3d10 4p4. The superscripts on the 4s and the 4p indicate the number of electrons in these orbitals. Add the superscripts on the 4s and the 4p.
Example 2: Determine the number of valence shell electrons in strontium (
38Sr, in yellow).
Answer: 2
Discussion: Strontium is in group 2. Its configuration is [Kr] 5s2. The superscripts on the 5s indicates the number of electrons in this orbital. That’s just two outer shell electrons. All other electrons are core electrons.
Example 3: Determine the number of valence shell electrons in thallium (
81Th, in purple).
Answer: 3
Discussion: Thallium is in group 13. Its configuration is [Xe] 6s2 4f14 5d10 6p1. The superscripts on the 6s and the 6p indicate the number of electrons in these orbitals. Add the superscripts on the 6s and the 6p.
Before You Leave
Check Your Understanding
Use the following questions to assess your understanding. Use this periodic table when needed. Tap the Check Answer buttons when ready.
1. How many elements are in any given row of the …
- s block?
- p block?
- d block?
- f block?
2. What is the maximum number of electrons that can be placed in all the …
- s orbitals of any given energy level?
- p orbitals of any given energy level?
- d orbitals of any given energy level?
- f orbitals of any given energy level?
3. Use
a periodic table to identify the element (by symbol or name) whose electron configuration ends with …
- 3s1
- 2p5
- 6d3
- 5s2
- 6p3
- 5f12
4. Who Am I?
Use
a periodic table to identify the element (by symbol or name) that fits the following descriptions.
- I am in period 2 and have exactly three electrons in my p orbitals.
- I am in perod 4 and have three valence electrons.
- My electron configuration ends with 5p4.
- When I form a 2+ ion, my electron configuration is identical to xenon’s.
- When I form a 2- ion, my electron configuration is identical to xenon’s.
- My electron configuration ends with 3d8.
- I am the lanthanide with 6 f orbital electrons.
- I have exactly 2 d orbital electrons.
- I am in the fourth period and have three d orbitals completely filled with two electrons.
- I am in period 6 and have 7 valence electrons.
5. Write the abbreviated electron configuration of …
- 13Al:
- 19K:
- 25Mn:
- 33As:
- 36Kr:
- 45Rh:
- 76Os:
- 93Np: