Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Elements and Compounds
Part a: The Periodic Table of Elements
Part 3a: The Periodic Table of Elements
Part 3b: Words, Symbols, and Particle Diagrams
What is an Element? a Compound?
It is an accepted belief that atoms are the building blocks of matter. There are (as of this writing) 118 different types of atoms, each differing from each other by their physical and chemical properties and some structural traits that will be discussed in the next chapter. These different types of atoms are referred to as elements. Examples of familiar elements include hydrogen (H), carbon (C), oxygen (O), nitrogen (N), sodium (Na), and chlorine (Cl). The letters in parenthesis are the elemental symbols associated with each of these elements.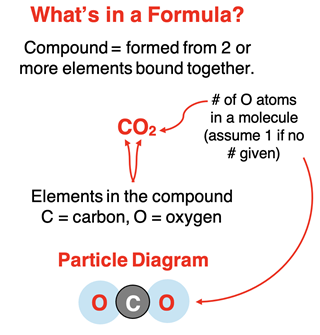
Atoms of different elements can combine with one another to produce compounds. Compounds you may be familiar with include dihydrogen monoxide (H2O, also known as water), carbon dioxide (CO2), sodium chloride (NaCl), and carbon monoxide (CO). The symbols in parenthesis are the chemical formulae of the compounds. Note that the chemical formula for each of these compounds includes two elemental symbols. This makes sense because a compound is composed of two or more different elements. The subscripted numbers indicate the number of atoms of each element in a single unit of the compound. The simplest unit is referred to as a molecule. There are two atoms of hydrogen and one atom of oxygen in a water molecule.
The Periodic Table of Elements
The Periodic Table may be the all-time greatest infographic. Information about each of the 118 elements is contained within the seven rows and 18 columns of the periodic table.
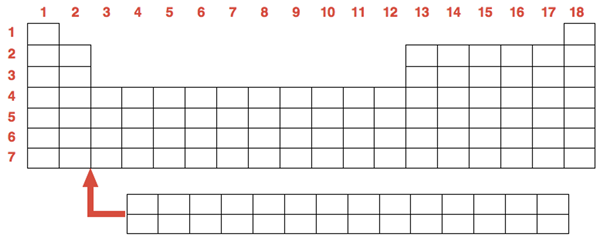
 Each box or cell of the periodic table represents an element. Each element is represented by an elemental symbol consisting of 1 or 2 letters. The first letter is always capitalized. The second letter (if applicable) is lower case. Most elemental symbols are relatively intuitive – H for hydrogen, He for helium, C for carbon, S for sulfur, etc. The symbols for some elements have their roots in Latin words and are less intuitive. These include iron (Fe for ferrum, meaning sword), lead (Pb for plumbum, indicating one of its earliest uses in plumbing), gold (Au for aurum, meaning shine), and mercury (Hg for hydragyrum, which means liquid silver)
Each box or cell of the periodic table represents an element. Each element is represented by an elemental symbol consisting of 1 or 2 letters. The first letter is always capitalized. The second letter (if applicable) is lower case. Most elemental symbols are relatively intuitive – H for hydrogen, He for helium, C for carbon, S for sulfur, etc. The symbols for some elements have their roots in Latin words and are less intuitive. These include iron (Fe for ferrum, meaning sword), lead (Pb for plumbum, indicating one of its earliest uses in plumbing), gold (Au for aurum, meaning shine), and mercury (Hg for hydragyrum, which means liquid silver)
Every element has a whole number (an integer) associated with it. This number is known as the atomic number. The elements of the table are ordered by their atomic number. This number increases by 1 as you progress from element to element across the table. We will discuss the significance of the atomic number in greater detail in Chapter 3 of the Chemistry Tutorial. Each cell of the periodic table also lists the atomic mass of each element. This is the relative mass of an atom of each element. This will be of great importance in later chapters of the Chemistry Tutorial.
Groups, Periods, Families
The unusual shape of the Periodic Table emerged from the observation that there were repeating patterns in the properties of elements. For instance, the size of the atoms of each element decreases as one proceeds across each row. And elements with similar properties (for instance the property of being inert or non-reactive) were placed in the same columns. The rows of the periodic table are referred to as periods. Each period is numbered; there are seven of them. The columns of the periodic table are referred to as groups. Each group is numbered from 1 to 18. There are 10 groups – groups 3-12 – which consist entirely of metals. These are referred to as Transition Metals. There are two rows of 14 elements each that are “pull-outs” from the table The top row of elements are called Lanthanides (atomic #57-#70). The bottom of these two rows are called Actinides (atomic #89-#102).
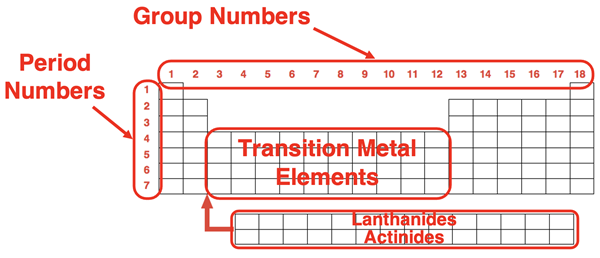
Because elements within the same group share similar physical and chemical properties, they are referred to as families. There are four notable families whose names you should commit to memory. Those families are:
- Alkali Metals (Group 1)
- Alkaline Earth Metals (Group 2)
- Halogens (Group 17)
- Noble Gases (Group 18)
Metals, Nonmetals, and Metalloids
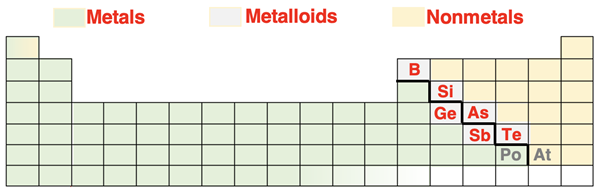
In Lesson 2, we mentioned that elements can be identified by their unique chemical and physical properties. Based on those properties, we can divide the 118 elements into two broad groups – metals and nonmetals. There are 6-8 elements (depending on the source) that share the properties of both metals and nonmetals. These elements are referred to as metalloids. The dividing line separating the metals from the nonmetals zigzags through the table as shown. There are six elements along the line that are considered to be metalloids by most sources. Their symbols are shown in red. Polonium (Po) and astatine (At) are the two elements for which there is the most disagreement regarding their classification as metalloids. Their symbols are shown in grey.
Some of the most common properties of metals and nonmetals are shown in the table below. While there are metal and nonmetal elements that display exceptions to some of these properties, the listing is generally fitting of metallic and nonmetallic elements.
Solids, Liquids, and Gases
Many periodic tables include some form of color-coding to indicate the state – solid, liquid, or gas – in which the elements naturally exist. Consistent with the property that metals have relatively high melting points, most metals are solids at room temperature. The exception is mercury (Hg) which is a liquid at room temperature. And at temperatures just above room temperature, gallium (Ga) will melt and form a liquid. Most nonmetals are gases. There are plenty exceptions to
the rule. For instance, you can find nonmetals that are solids (carbon, phosphorus, sulfur, selenium, iodine, and more) and liquids (bromine).
Elemental Forms
If you acquire a sample of pure copper, all the atoms in the sample will be copper atoms. And these copper atoms exist as single atom particles. If you acquire a sample of pure helium gas, all the atoms will be helium and the helium atoms will exist as single atom particles. We refer to copper and helium as being
monatomic – single atom. Most elements exist naturally as monatomic elements. But not all.

There are seven elements on the periodic table that exist naturally as diatomic elements. These elements, when found in nature by themselves, contain particles with two atoms of each element. We refer to these particles as
diatomic molecules. The seven elements are hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, and iodine. When a sample of oxygen is found as an isolated element in nature, it exists as two atoms of oxygen bound together to form the O
2 molecule. You can remember these seven elements if you remember the name of one of our favorite college Chemistry professors – Dr.
HONClBrIF.
Finally, there are a few other elements that exist in
polyatomic form in nature. The most notable of these is phosphorus and sulfur. Phosphorus is found in nature as P
4 and sulfur is found in nature as S
8.
 The Alphabet of Chemistry
The Alphabet of Chemistry
The English language has 26 letters in its alphabet. These 26 letters can be combined together to form words. Different words are formed by varying the number of letters and the types of letters that we combine. It is estimated that anywhere between 600 000 and one million words can be formed from these 26 letters of the alphabet.
The elemental symbols of the periodic table are like the alphabet of Chemistry. These 118 symbols can be combined together to form chemical formulas for compounds. By varying which symbols (and elements) that you combine and how many atoms of each element you

combine, you can produce different chemical formulae for compounds. While not every combination is possible (and we will learn why in the next few units), the number of compounds that are currently known is in the millions.
In the
next part of Lesson 3, we will begin building a more thorough understanding of chemical formulae and relate that understanding to our growing vocabulary associated with matter – atoms, molecules, elements, and compounds.
Before You Leave
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. How many …
- … transition metals are located in Group 4?
- … transition metals are located in Period 4?
- … nonmetals are located in Period 3?
- … metalloids are located in Group 14?
- …halogens have a mass greater than bromine?
- … noble gases have atomic numbers less than 36?
- … transition metals have an atomic number greater than 74?
- … noble gases are in the same period as a transition metal?
2. What is/are the symbol(s) of …
- … the element in Period 4 of the halogen family?
- … the element in Period 3 of the alkaline earth metal family?
- … the element in Period 3, Group 4?
- … the element in Period 4, Group 3?
- … the element in Period 3, Group 17?
- … the element in Period 6, Group 13?
- … the element with an atomic number that is 4 greater than the Period 3 alkali metal?
- … the elements that are noble gases and have a mass less than the first transition metal?
- … the Period 4 metalloids?
- … all the nonmetal elements in Period 3?
- … all the Period 6 gases?
3. Ken Fused is pondering the concept of elements that are diatomic. He observes that carbon monoxide has a chemical formula of CO. Confused, he asks
Why shouldn’t there be a 2 after the O in the formula since oxygen is one of the diatomic elements?
Help straighten Ken’s thinking out by explaining what is meant by a diatomic element and why there doesn’t need to be a 2 in the compound’s formula.