Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Elements and Compounds
Part b: Words, Symbols, and Particle Diagrams
Part 3a: The Periodic Table of Elements
Part 3b: Words, Symbols, and Particle Diagrams
Language Lesson – Elements, Atoms, and Molecules
One of the challenges for the student of Chemistry is to learn the language. Words like element and compound and atom or molecule have precise meaning and must be used correctly. Let’s have a language lesson!
Matter is composed of atoms - the individual units that form the building blocks from which all things are made. There are many types of atoms, each with a different name (like copper or aluminum or oxygen or nitrogen). These types of atoms are referred to as elements. Elements can consist of individual atoms or of groups of the same type of atoms bound together. When two or more atoms bind together to form a multi-atom particle, we refer to the particle as being a molecule. Particle diagrams like the ones below often help students to keep the language straight.
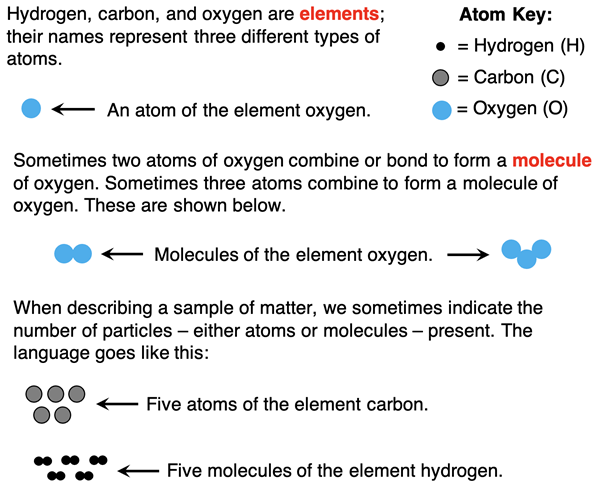
Language Lesson – Compounds and Molecules
We saw in the diagram above that two or even three oxygen atoms can bond together to form a molecule of oxygen. But molecules can also be formed when groups of atoms of different elements bond. When atoms of two or more elements bind together, a compound is formed. For instance, water (H2O) is a compound formed when two atoms of hydrogen bind to one atom of oxygen. We refer to this grouping of three atoms as a molecule of water.
Think of a molecule as a particle that contains two or more atoms bound together. If all the atoms in a molecule are the same type, then it is a molecule of an element (O2, O3, N2, etc.). If the atoms in a molecule are of different types, then it is a molecule of a compound (CO, CO2, H2O, H2O2, etc.). Think of a compound as a type of substance that contains two or more elements. Once again, particle diagrams like the ones below can be very helpful.
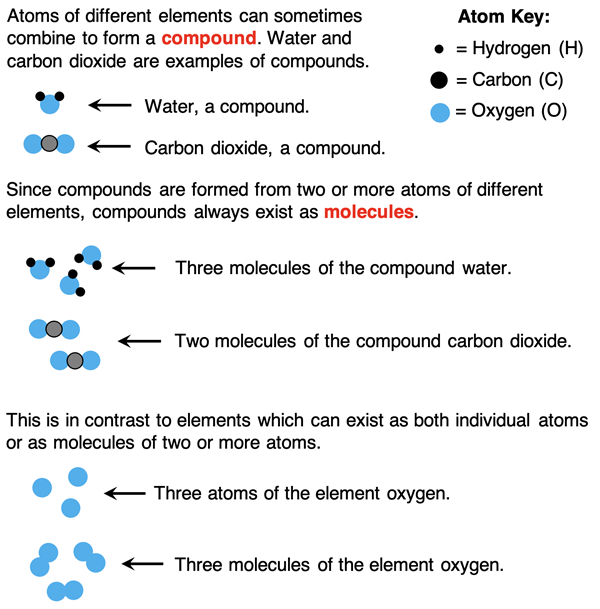
 In Chemistry, we will frequently talk of particle-level ideas. That’s the small stuff. And we will also talk of the macroscopic. That’s the big stuff … the observables. In our language of Chemistry, atoms and molecules are used to refer to particles or the individual units that make up a sample of matter. Elements and compounds are used to refer to the sample of matter. The language can be confusing. But it will grow on you. Like any language, you will need to practice using it – reading about it, listening to it, and speaking it.
In Chemistry, we will frequently talk of particle-level ideas. That’s the small stuff. And we will also talk of the macroscopic. That’s the big stuff … the observables. In our language of Chemistry, atoms and molecules are used to refer to particles or the individual units that make up a sample of matter. Elements and compounds are used to refer to the sample of matter. The language can be confusing. But it will grow on you. Like any language, you will need to practice using it – reading about it, listening to it, and speaking it.
Symbolic Representations of Matter
 Fluency in Chemistry class requires more than being fluent in the language of Chemistry. You must also be fluent in the use of symbols to represent chemical systems. Every element has its own elemental symbol – like H, C, O, N, Na, He, etc. And every compound can be represented by a chemical formula – a collection of symbols and numbers and parenthesis (if you’re lucky). You’ll need to be able to make sense of formulas such as H2O, CO2, AlCl3, HNO3, (NH4)2SO4, and so on. As time continues, you will see complex formula expressions like Pb(NO3)2 + 2 KI.
Fluency in Chemistry class requires more than being fluent in the language of Chemistry. You must also be fluent in the use of symbols to represent chemical systems. Every element has its own elemental symbol – like H, C, O, N, Na, He, etc. And every compound can be represented by a chemical formula – a collection of symbols and numbers and parenthesis (if you’re lucky). You’ll need to be able to make sense of formulas such as H2O, CO2, AlCl3, HNO3, (NH4)2SO4, and so on. As time continues, you will see complex formula expressions like Pb(NO3)2 + 2 KI.
Nobody is born with an understanding of these symbolic representations. Learning them will take some time. And some practice. And some instruction; and that’s what this Chemistry Tutorial is for.
Making Sense of Symbols
The elemental symbols used in formulae (plural form of formula) indicate what types of atoms are present. The numbers are either coefficients or subscripts and they indicate the number of atoms or molecules present.
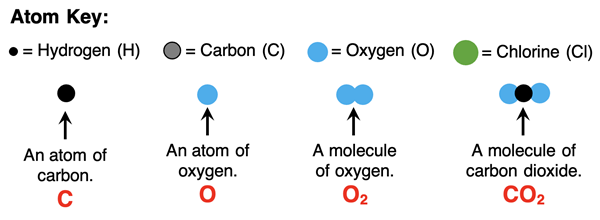
Subscripts are used to indicate the number of each atom present. The 2 in CO2 indicates there are two atoms of oxygen in the molecule. If you don’t see a subscript, then you can assume there is just 1 atom.
Here are more examples using the same Atom Key shown above:
So, the subscripts in formulas indicate the number of atoms of each element in a molecule. Formulas also include numbers known as coefficients. The red numbers in the formulas below are examples.
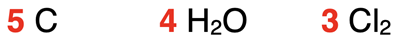
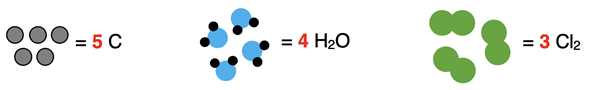
The coefficient 5 as in 5 C indicates that there are five of whatever symbol it comes in front of – in this case, there are five C atoms. The 4 of 4 H2O indicates that there are four water molecules. And the 3 of 3 Cl2 indicates that there are three chlorine molecules. The particle diagrams would look like this:
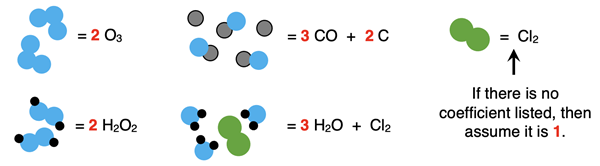
Here are some more examples of formulas containing coefficients:
Parentheses are sometimes included in the chemical formulas for a type of compound known as an ionic compound. Here are three examples:

 There is always a subscripted number after a parenthesis. A subscript of 2 means that there are 2 copies of whatever is inside of the parenthesis. We will discuss formula writing for ionic compounds in great detail in Chapter 4 of this Chemistry Tutorial.
There is always a subscripted number after a parenthesis. A subscript of 2 means that there are 2 copies of whatever is inside of the parenthesis. We will discuss formula writing for ionic compounds in great detail in Chapter 4 of this Chemistry Tutorial.
Counting Atoms from Symbolic Representations
In Lesson 1, we discussed how Chemistry was a quantitative science. Numbers matter. And many concepts have a numerical slant. Symbolic representations are one of many demonstrations of the numerical nature of Chemistry. Subscripts and coefficients allow us to count the number of atoms of each element represented by a formula. Consider the atom counts shown in the table below for a variety of formulas.
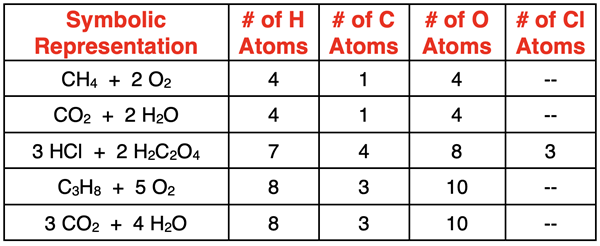
Sense-Making Tools
This page has discussed three related tools – language, symbolic representations, and particle diagrams. These are tools. Like any tool, you don’t use it once and throw it away. The best tools are those that you use all the time. In Chemistry, language, symbolic representations, and particle diagrams will be our frequently used tools to for making sense of Chemistry. Don’t throw these tools away! You’re going to use them all the time.
Before You Leave
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Identify the following particle diagrams as representing either an pure element, a pure compound, a mixture of two elements, a mixture of two compounds, or a mixture of an element and a compound.
2. Classify the following formulae as representing either a pure element, a pure compound, a mixture of two elements, a mixture of two compounds, or a mixture of an element and a compound.
- 3 H2O
- 3 Zn + 2 H2
- 3 H2C2O4
- C8H18
- 2 C8H18 + 17 O2
- S8
3. Use the language of Chemistry to describe the following particle representations.
4. Explain how the symbols
2 O3 is different than
3 O2.
5. Use a symbolic representation to describe the following particle representations.
6. Fill in the table, indicating the number of atoms of each indicated element.
7. Rank the following samples in terms of which has the greatest number of oxygen atoms (O), from the least number to the greatest number.