Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Classifying Chemical Reactions
Part a: Decomposition and Synthesis Reactions
Part a: Decomposition and Synthesis Reactions
Part b:
Combustion Reactions
Part c:
Single Replacement Reactions
Part d:
Double Replacement Reactions
Part e:
Predicting Products

Reaction Types
There are a variety of ways of classifying types of chemical reactions. Lesson 2 will focus on five different reaction types. The five types are synthesis, decomposition, combustion, single replacement, and double replacement. Nearly every reaction will fit into at least one of these types.
Being able to identify a reaction as being of a particular type allows you to use patterns of chemical behavior to predict the products of a reaction. The focus of Lesson 2 will be on using information about reaction types as a guide to predicting the products of a reaction.
Synthesis Reactions (a.k.a., Combination Reactions)
In a synthesis reaction, two or more substances combine to form a single, more complex substance. A synthesis reaction is sometimes referred to as a combination reaction. The product of a synthesis reaction is always a compound. The reactants of a synthesis reaction can be two elements, an element and a compound, or even two simple compounds. If the product is a binary ionic compound, then the reactants are a metallic element and a nonmetal element. If the product is a binary molecular compound, then the reactants are two nonmetal elements.
 Sodium chloride (NaCl) is a binary ionic compound. The synthesis reaction for the formation of sodium chloride is …
Sodium chloride (NaCl) is a binary ionic compound. The synthesis reaction for the formation of sodium chloride is …
2 Na(s) + Cl2(g) → 2 NaCl(s)
You know that this is a synthesis reaction because there is only one product.
Iron reacts with chlorine to form a binary ionic compound. This would be a synthesis reaction. Iron is a transition metal and the actual charge of iron in the ionic compound is difficult to predict. Let’s suppose it forms a 3+ ion with the chloride ion. The synthesis of iron(III) chloride is represented by the chemical equation …
2 Fe(s) + 3 Cl2(g) → 2 FeCl3(s)
Sulfur trioxide (SO3) is a binary molecular compound. It is produced by the reaction of sulfur and oxygen. The balanced chemical equation is …
2 S(s) + 3 O2(g) → 2 SO3(g)
This is clearly a synthesis reaction because of the presence of a single product. In Lesson 2b, we will learn that this is also categorized as a combustion reaction. It is not uncommon that a reaction falls into two different reaction type categories.
Nonmetals such as sulfur and oxygen can often combine to produce different products. The most common products for these two nonmetals are SO2 and SO3.
Here are the balanced chemical equations for a few other synthesis reactions: Observe that there is a single product in each balanced chemical equation. The reactants are always simpler substances (containing less atoms) than the product. As can be seen in the last three equations, the reactants are not restricted to being elements.
3 Mg(s) + N2(g) → Mg3N2(s)
2 Cu(s) + S(g) → Cu2S(s)
Cu(s) + S(g) → CuS(s)
2 H2(g) + O2(g) → 2 H2O(l)
2 CO(g) + O2(g) → 2 CO2(g)
MgO(s) + CO2(g) → MgCO3(s)
CaO(s) + H2O(l) → Ca(OH)2(aq)
Predicting Products (and Reactants) for Synthesis Reactions
A beginning student of chemistry can easily acquire the skill of predicting the product formula for a simple synthesis reaction. Similarly, the reactant formulae (plural for formula) for the synthesis of a given binary compound can also be identified. And once reactant and product formulae are identified, the balanced chemical equation can be written. These tasks require an understanding of …
- … the nature of a synthesis reaction.
- … formula writing for binary ionic compounds and binary molecular compounds.
- … the seven elements that exist as diatomic molecules.
- … the method of writing a balanced chemical equation.
Here are four example questions. See if you can answer them on your own. Utilize the links above if needed. Tap the
View Answer button to view the answer and the explanation.
Example 1: Solid aluminum reacts with chlorine gas in a synthesis reaction. Predict the product. Write the skeleton equation (proper formulae, no coefficients). Then add coefficients to balance the chemical equation.
Example 2: Hydrogen gas reacts with chlorine gas in a synthesis reaction. Predict the product. Write the skeleton equation (proper formulae, no coefficients). Then add coefficients to balance the chemical equation.
Example 3: Nitrogen trioxide gas is synthesized from its elements. Identify the reactants. Write the skeleton equation (proper formulae, no coefficients). Then add coefficients to balance the chemical equation.
Example 4: Solid titanium(IV) nitride is synthesized from its elements. Identify the reactants. Write the skeleton equation (proper formulae, no coefficients). Then add coefficients to balance the chemical equation.
Decomposition Reactions
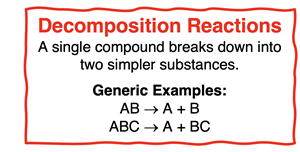
In a
decomposition reaction, a complex compound is broken down (
decomposes) into two or more simpler substances. There is only one reactant in a decomposition reaction and there are two or more products. Saying a reactant is
a complex compound simply infers that it contains relatively more atoms than any of the individual products.
Motivating a compound to decompose usually requires the application of heat or electricity.
If the reactant is a
binary ionic compound, then the products will be elements - a metal and a nonmetal. If the reactant is a
binary molecular compound, then the products will usually be two nonmetal elements. There are exceptions as you will see in the examples below. If the reactant is composed of three elements, the products are less predictable; they are usually a compound and an element.
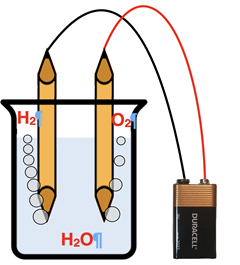
Water is a
binary molecular compound. When electricity is applied to a sample of water, it decomposes into its elements – hydrogen and oxygen. The balanced chemical equation for the decomposition of water is …
2 H2O(l) → 2 H2(g) + O2(g)
Note that this balanced equation for the decomposition of water is the opposite of the balanced equation for the synthesis of water from its elements:
2 H2(g) + O2(g) → 2 H2O(l)
A decomposition reaction has a form that is opposite of a synthesis reaction. In synthesis reactions, there are two or more reactants and one product. In decomposition reactions, there is one reactant and two or more products.
Mercury(II) oxide is a
binary ionic compound composed of the elements mercury and oxygen. Upon heating, it decomposes into its elements. Mercury is the lone exception of a metal that is a liquid. Oxygen is a diatomic element; like most nonmetals, it is a gas. The balanced chemical equation for the decomposition of mercury(II) oxide is:
2 HgO(s) → 2 Hg(l) + O2(g)
Balanced chemical equations for a few other decomposition reactions are shown below. Observe that there is a single reactant in each balanced chemical equation. The reactants are always more complex substances, containing more atoms than any individual product.
2 H2O2(l) → 2 H2O(l) + O2(g)
CaCO3(s) → CaO(s) + CO2(g)
H2CO3(aq) → CO2(g) + H2O(l)
Predicting Products (and Reactants) for Decomposition Reactions
Predicting the products for a decomposition reaction can involve a bit more guesswork than for synthesis reactions. With some subtle hints, it is very possible to do. Like synthesis reactions, identifying the products and writing a balanced chemical equation for a decomposition reaction requires some prerequisite knowledge. This includes an understanding of formula writing and
equation balancing.
Here are two example questions. See if you can answer them on your own. Tap the
View Answer button to view the answer and the explanation.
Example 5: Solid aluminum oxide decomposes into its elements. Identify the reactant and product formulae, write the skeleton equation (proper formulae, no coefficients), then add coefficients to balance the chemical equation.
Example 6: At high temperatures, gaseous ammonia (NH
3) decomposes into its elements. Identify the reactant and product formulae, write the skeleton equation (proper formulae, no coefficients), then add coefficients to balance the chemical equation.
Before You Leave
- Download our Study Card on Types of Reactions. (It covers Lessons 2a, 2b, 2c, and some of Lesson 2d.) Save it to a safe location and use it as a review tool.
- Once you have some comfort with Lessons 2a through 2d, try our Chemical Reaction Type Concept Builder. It will provide awesome practice on all five reaction types.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Describe how you can tell the difference between a synthesis reaction and a decomposition reaction.
2. Classify the following reactions as being either synthesis, decomposition, or neither.
- A + CD → ACD
- ACD → AD + C
- A + CD → AD + C
- AB + CD → ABCD
3. Classify the following reactions as being either synthesis, decomposition, or neither.
- 2 FeO(s) → 2 Fe(s) + O2(g)
- 2 Al(s) + N2(g) → 2 AlN(s)
- MgCl2(s) + F2(g) → MgF2(s) + Cl2(g)
- Zn(s) + S(s) → ZnS(s)
- 2 Fe(OH)3(s) → Fe2O3(s) + 3 H2O (g)
4. For the following situations, identify the formulae for reactants and products, write the skeleton equations, and then add coefficients to balance the chemical equation.
- Na(s) + O2(g) → ????
- ??? + ??? → Fe3N2(s)
- Copper reacts with sulfur to form an ionic compound. Assume copper forms a 1+ ion.
- Electric current is passes through a sample of sodium chloride. It decomposes into its elements.