Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Matter and Its Classification
Part b: Pure Substances vs. Mixtures
Part 1a: Solids, Liquids, and Gases
Part 1b: Pure Substances vs. Mixtures
 What is this Stuff?
What is this Stuff?
One of the key roles of chemistry is to identify the various materials of our world. Chemists are matter detectives. They are often given a sample of a material – a solid, a liquid, or a gas - and asked to determine what it is. What is it made of? How can its composition be described? Chemists rely on a convenient classification system for answering these questions. But before we can understand the system, we need to do engage in some small talk about atoms.
Atoms, Elements, and Compounds
For more than a couple of centuries, scientists have been quite comfortable with the concept of the atom as the building block of matter. Not all atoms are equal. We have learned that there are different types of atoms. As of this writing (2024), we have been able to detect 118 unique types of atoms. We refer to these types of atoms as elements.
You’re likely familiar with the names many of these elements – hydrogen, helium, oxygen, sodium, mercury, aluminum, copper, and many more. Each of these types of atoms (i.e., elements) differ from each other in terms of their observable properties. For instance, hydrogen is a colorless, odorless, flammable gas. Sodium is a soft, silvery-white solid that reacts with water. Mercury is a dense, silver liquid with a strong tendency to ball up into beads. And copper is a reddish-brown, highly conductive solid that is often used for electrical wiring. While these elements have unique observable properties that allow chemists to identify them, there are also distinct differences at the particle level that make an atom of copper distinguishable from an atom any of the other existing element. Atoms and elements will be discussed in great detail as we continue through this Chemistry Tutorial.

Tap for Photo Credits: Copper || Sodium || Mercury
Atoms of different elements often combine through chemical reactions to produce compounds. A compound is a type of substance that is composed of atoms of two or more elements. Water is an example of a compound. Water consists of hydrogen atoms and oxygen atoms. In our nerdiest of moments, we chemistry types call water dihydrogen monoxide and represent it symbolically as H2O. Examples of other compounds include carbon dioxide (produced by burning fossil fuels), carbon monoxide (a poisonous gas), sodium chloride (table salt), silicon dioxide (sand), and iron oxide (rust). Compounds will be the focus of our study in Chapter 4 of this Chemistry Tutorial.
A Classification System
That’s enough small talk. Let’s answer the big question:
When you look at the stuff that surrounds you, how do you know what it is made of? In terms of its parts, how can you describe the composition of stuff?
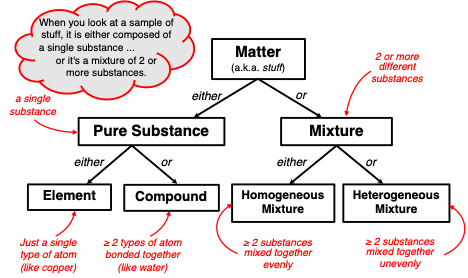
Chemists approach this question using the classification system depicted in the graphic below. A sample of matter is one of two things – it is either a pure substance (that is, a single substance) or it is a mixture of two or more substances. A
pure substance contains only one type of substance. That type of substance could be an
element like copper (Cu), helium (He), oxygen (O), nitrogen (N), or any of the 118 elements on the periodic table. Or that type of substance could be a
compound like water (H
2O), carbon dioxide (CO
2), carbon monoxide (CO), and sodium chloride (NaCl). If the sample of matter is not a pure substance, then it is a
mixture. If the different substances of a mixture are uniformly spread throughout the bulk of the sample, then the mixture is referred to as a
homogenous mixture. In
heterogeneous mixtures, the individual substances are either separated or non-uniformly mixed.
Mixtures
 In nature, mixtures are far and away more common than pure substances. Air is a mixture of several gases, primarily nitrogen (~78%) and oxygen (~21%). Tap water is mostly water but also includes a variety of other substances mixed in; these include minerals (such as sodium, iron, calcium, and magnesium), chlorine-containing compounds, and other trace impurities. Air and tap water are both homogeneous mixtures because the components or parts of the mixture are spread evenly throughout the sample of air and of water. Black coffee, cologne, and vinegar are other examples of homogeneous mixtures. If you inspect the sample of these mixtures, it will be hard to see the individual components because they are evenly spread throughout. Being mixed together uniformly, the parts are indistinguishable from one another.
In nature, mixtures are far and away more common than pure substances. Air is a mixture of several gases, primarily nitrogen (~78%) and oxygen (~21%). Tap water is mostly water but also includes a variety of other substances mixed in; these include minerals (such as sodium, iron, calcium, and magnesium), chlorine-containing compounds, and other trace impurities. Air and tap water are both homogeneous mixtures because the components or parts of the mixture are spread evenly throughout the sample of air and of water. Black coffee, cologne, and vinegar are other examples of homogeneous mixtures. If you inspect the sample of these mixtures, it will be hard to see the individual components because they are evenly spread throughout. Being mixed together uniformly, the parts are indistinguishable from one another.
 A homogeneous mixture is often referred to as a solution. A solution has a solvent and one or more solutes. The solvent is generally thought of as the substance in the solution that is present in the greatest amount. The solute is dissolved into the solvent and uniformly distributed throughout. If a teaspoon of table salt (NaCl) is added to a glass of water and stirred, the salt will dissolve in the water to form a solution. The water is the solvent, and the salt is the solute. As you will soon learn, water is a commonly used solvent in Chemistry class. Various solids and on occasion liquids and gases will be dissolved into water to form an aqueous solution. Coffee, vinegar, and contact lens solution are all examples of aqueous solutions encountered in your everyday life.
A homogeneous mixture is often referred to as a solution. A solution has a solvent and one or more solutes. The solvent is generally thought of as the substance in the solution that is present in the greatest amount. The solute is dissolved into the solvent and uniformly distributed throughout. If a teaspoon of table salt (NaCl) is added to a glass of water and stirred, the salt will dissolve in the water to form a solution. The water is the solvent, and the salt is the solute. As you will soon learn, water is a commonly used solvent in Chemistry class. Various solids and on occasion liquids and gases will be dissolved into water to form an aqueous solution. Coffee, vinegar, and contact lens solution are all examples of aqueous solutions encountered in your everyday life.
While homogeneous means the same throughout, heterogeneous means widely varied or unevenly distributed. A heterogeneous sample of anything is a sample in which the parts or components are distributed unevenly throughout the sample. A bowl of chili or a pepperoni pizza are examples of heterogeneous mixing of the parts of a sample.

 Oil and vinegar salad dressing and orange juice (with pulp) are examples of heterogeneous mixtures found in your refrigerator. Before pouring these mixtures from a container, you are typically advised to shake it. Shaking will distribute or mix the parts of the mixture more evenly. When you remove a sample of salad dressing or OJ from the container, you are removing a representative mix of its components. But if given enough time, the components will once again separate into two phases and return to the state of being unevenly distributed.
Oil and vinegar salad dressing and orange juice (with pulp) are examples of heterogeneous mixtures found in your refrigerator. Before pouring these mixtures from a container, you are typically advised to shake it. Shaking will distribute or mix the parts of the mixture more evenly. When you remove a sample of salad dressing or OJ from the container, you are removing a representative mix of its components. But if given enough time, the components will once again separate into two phases and return to the state of being unevenly distributed.
Pure Substances
A sample of matter that is classified as a pure substance has only one component in it. It is a single substance. Pure. Not mixed with any other substance. Uncontaminated by any other substance in its vicinity. Such samples are difficult to find in nature since the side-by-side presence of any two substances often results in their intermingling or mixing.
Let’s do a thought experiment. Suppose you line the bottom of a pan with rocks and then pour the purest sample of water you can find on top of it. Over time, minerals in the rock will dissolve into the water. Gases in the air above the water surface will dissolve in the water. Soon the purest sample of water you could find becomes a mixture with gases and minerals spread throughout.
 So, if pure substances are nearly non-existent in nature, then how can we ever acquire pure substances? Great question. Answer: We rely on chemistry for better living. The chemical industry spends lots of time separating the components of a mixture from one another in order to create pure samples of a single substance. Rocks are mined and the minerals inside them are separated and refined to produce pure samples of elements like aluminum, iron, copper, etc. Similar processes are used to acquire pure samples of compounds such as sodium bicarbonate (baking soda), table sugar (sucrose), and sodium chloride. The chemical industry spends time and effort acquiring samples of pure gases like helium, nitrogen, and oxygen. And water solutions are purified to produce pure water.
So, if pure substances are nearly non-existent in nature, then how can we ever acquire pure substances? Great question. Answer: We rely on chemistry for better living. The chemical industry spends lots of time separating the components of a mixture from one another in order to create pure samples of a single substance. Rocks are mined and the minerals inside them are separated and refined to produce pure samples of elements like aluminum, iron, copper, etc. Similar processes are used to acquire pure samples of compounds such as sodium bicarbonate (baking soda), table sugar (sucrose), and sodium chloride. The chemical industry spends time and effort acquiring samples of pure gases like helium, nitrogen, and oxygen. And water solutions are purified to produce pure water.
Before You Leave
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Can an element – a single type of atom – ever be part of a mixture?
2. Based on the chemical name that is given, identify the following as either an
element or a
compound:
a. Hydrogen chloride
b. Phosphorus
c. Uranium
d. Phosphorus pentachloride
e. Aluminum sulfide
3. Classify the following mixtures as being either a homogeneous mixture or a heterogeneous mixture.
a. Fruit Salad
b. Glass of ice water
c. Steel
d. Chocolate chip cookie
e. Milk
f. Tap water
4. Consider the samples of matter in each of these containers. Classify each as being either a pure substance, a homogeneous mixture, or a heterogeneous mixture.
