Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Describing a Gas
Part a: The Nature of a Gas
Part a: The Nature of a Gas
Part b:
Pressure-Volume-Temperature-Moles
Solids, Liquids, and GASES
We introduced states of matter in Chapter 2. The three most common states of matter on Earth are solids, liquids, and gases. In Chapter 10, we will focus on matter in the gaseous state. In Lesson 1, we will discuss how we describe the gaseous state.
As we have learned, a solid is characterized as having a fixed shape and a fixed volume. A pencil is an example of a solid object. Whether the pencil is in your backpack, on your desk, or in your hand, its shape and its volume are unchanged. Like any solid, it has a shape and a volume that is independent of the container that contains it.
A liquid is characterized as having a variable shape and a fixed volume. Consider the liquid H2O that partially fills your plastic water bottle. The shape assumed by the water is determined by the shape of the water bottle. But the volume of the water is independent of the bottle. The water can be poured from the bottle into a small dish. It’s shape changes, but the volume of water remains the same. Spill the water on the floor and it assumes another shape; but it is still the same volume of water. Like any liquid, the shape of the water is variable and dependent on the container that contains it while the volume is fixed.
 In contrast to solids and liquids, a gas fills the container that it is in. The shape of the gas and the volume of the gas are determined by the container that contains it. Gases can be transferred from one container to another container, and the shape and the volume change as a result of the transfer. The air inside your lungs assumes the shape and volume of your lungs. Exhale a portion of that air into a balloon and the shape and volume both changes. Then empty the air of the balloon into the room. The gas expands and fills the room, thus assuming a new shape and a new volume. Gases have both a variable shape and a variable volume that is dependent on the container that contains it.
In contrast to solids and liquids, a gas fills the container that it is in. The shape of the gas and the volume of the gas are determined by the container that contains it. Gases can be transferred from one container to another container, and the shape and the volume change as a result of the transfer. The air inside your lungs assumes the shape and volume of your lungs. Exhale a portion of that air into a balloon and the shape and volume both changes. Then empty the air of the balloon into the room. The gas expands and fills the room, thus assuming a new shape and a new volume. Gases have both a variable shape and a variable volume that is dependent on the container that contains it.
We must ask, what is it about a gas that gives it this ability to expand to the boundaries of its container? Why is it so different than the solid state and the liquid state? Of course, there are answers to these questions. And those answers take us down to the particle level. Let’s now consider the gaseous state in terms of the particles that it is composed of.
Gases are a Non-Condensed State
The solid and liquid states are considered condensed states of matter. Particles are close together and particles interact with one another by exerting attractive forces upon one another. In solids, these interparticle attractions are rather strong. They are relatively weaker, yet still influential for the average liquid.
A gas in not a condensed state. Compared to solids and liquids, the distance between particles in a gas are considerably larger. This is quite noticeable if we compare the densities of a variety of substances in their liquid state and their gaseous state (at 0°C and 1 atm of pressure). Such data is shown in the table.
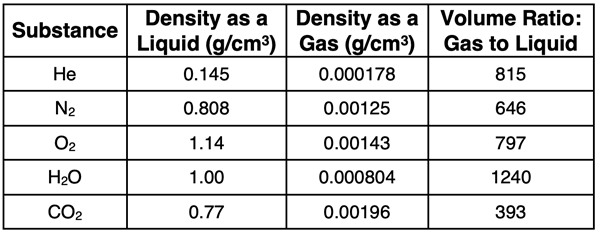
A change of state does not involve a change of mass. So, the difference in density values are due to the volume changes. The last column of the table shows the ratio of volume as a gas compared to that as a liquid. There is a considerable change in volume when a liquid changes to a gas. This is evidence for larger interparticle distances in the gaseous state.
In the liquid state, the distance between particles is about 1.1 to 1.3 times the size of the particle. For the gaseous state, the distance between particles depends upon pressure and temperature conditions. If we consider oxygen gas at standard conditions of 0°C and 1 atm, the average distance between O2 molecules is approximately 30 times the size of the particle.
 Not only are particles spaced further apart in the non-condensed gaseous state, but the attractive forces between particles are also considerably weaker. In the condensed solid state, the attractive forces are strong enough to hold particles in a rigid structure. In the condensed liquid state, the inter-particle attractions are strong enough to prevent the particles from escaping the herd and filling the entire container. But in the gaseous state, the interparticle attractions are so weak that we consider them to have a negligible effect upon the behavior of the gas. The result is that gases expand to fill the entire volume of the container that contains it. We will revisit this concept in more detail in Lesson 4 of this chapter.
Not only are particles spaced further apart in the non-condensed gaseous state, but the attractive forces between particles are also considerably weaker. In the condensed solid state, the attractive forces are strong enough to hold particles in a rigid structure. In the condensed liquid state, the inter-particle attractions are strong enough to prevent the particles from escaping the herd and filling the entire container. But in the gaseous state, the interparticle attractions are so weak that we consider them to have a negligible effect upon the behavior of the gas. The result is that gases expand to fill the entire volume of the container that contains it. We will revisit this concept in more detail in Lesson 4 of this chapter.
Gases are Compressible
Unlike solids and liquids, samples of gas are considered to be highly compressible. By applying pressure to the gas sample, its volume can be reduced. This trait of changing volume in response to changing pressure is known as compressibility.
 Gases are compressible while solids are not compressible and liquids have minimal compressibility. Find a textbook on a bookshelf. Put it on a table and press on it with all your might. It’s a solid. It’s not compressible. Jump off a high dive at a pool and land on the surface of the water with a bellyflop or spread eagle orientation. The liquid water will hardly give because it has a low compressibility. The pool water may splash since the particles are held together by weaker forces than in solids … but there won’t be a lot of compression and your body will provide the evidence for this.
Gases are compressible while solids are not compressible and liquids have minimal compressibility. Find a textbook on a bookshelf. Put it on a table and press on it with all your might. It’s a solid. It’s not compressible. Jump off a high dive at a pool and land on the surface of the water with a bellyflop or spread eagle orientation. The liquid water will hardly give because it has a low compressibility. The pool water may splash since the particles are held together by weaker forces than in solids … but there won’t be a lot of compression and your body will provide the evidence for this.
The absent or low compressibility of solids and liquids is due to the strong interparticle forces and the small interparticle distances. But gases have weak to no interparticle forces and large interparticle distances. The particles of a gas sample can be pressed closer together by the application of pressure. There is space between particles to do the compressing and there are no interparticle forces to prevent it.
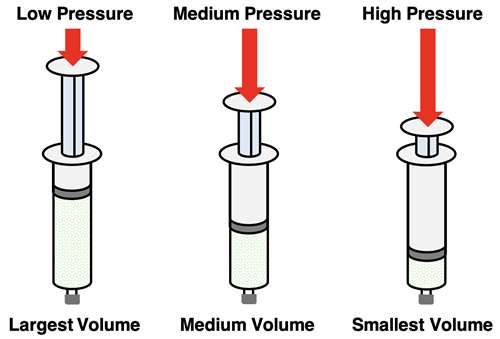
 The apparatus shown at the right is commonly used in Chemistry labs to demonstrate the compressibility of gases. A plastic, air-filled syringe is sealed at the bottom. A plunger is inserted into the barrel of the syringe to seal off the top. Pressure exerted upon the plunger of the syringe is transmitted as pressure exerted upon the gas sample. As more pressure is applied to the plunger, the volume of the gas sample decreases. The gas particles are pressed closer together space taken up by the gas is reduced. This demonstrates the property of compressibility - the application of external pressure reduces the volume of the gas sample.
The apparatus shown at the right is commonly used in Chemistry labs to demonstrate the compressibility of gases. A plastic, air-filled syringe is sealed at the bottom. A plunger is inserted into the barrel of the syringe to seal off the top. Pressure exerted upon the plunger of the syringe is transmitted as pressure exerted upon the gas sample. As more pressure is applied to the plunger, the volume of the gas sample decreases. The gas particles are pressed closer together space taken up by the gas is reduced. This demonstrates the property of compressibility - the application of external pressure reduces the volume of the gas sample.
Gas Particles are On the Move
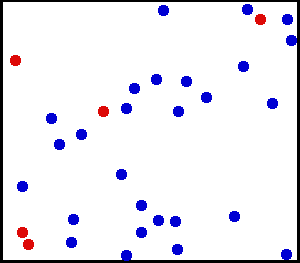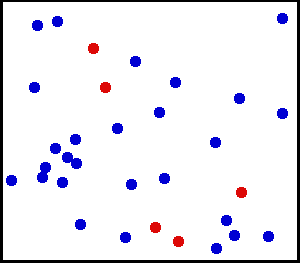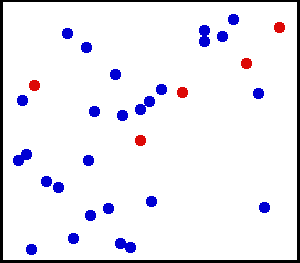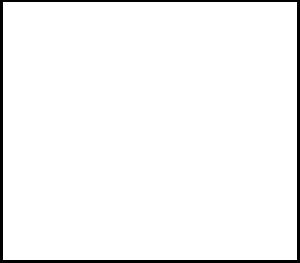 The particles in a sample of gas are in constant, random motion about the container. They continue moving until they encounter the walls of the container or another particle crossing its path. Upon meeting up with a container wall or another particle, they collide and change directions. This constantly-on-the-move behavior explains why gases expand to fill the entire volume of the container. And the collisions with the container walls are the source of gas pressure. (Animation: Public Domain)
The particles in a sample of gas are in constant, random motion about the container. They continue moving until they encounter the walls of the container or another particle crossing its path. Upon meeting up with a container wall or another particle, they collide and change directions. This constantly-on-the-move behavior explains why gases expand to fill the entire volume of the container. And the collisions with the container walls are the source of gas pressure. (Animation: Public Domain)
The speed at which gas particles move is dependent upon the temperature and the molar mass of the gas. Speeds increase with increasing temperature. And gases with larger molar mass values move slower than less massive particles when at the same temperature.
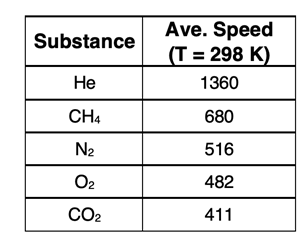
At any given moment, not all particles within the sample are moving at the same speeds. There is a range of speeds at which the gas particles move. This range is explained by the collisions that occur within the three-dimensional space of the container. This is illustrated in the animation above. Some particles move very slow, at least until the next collision. Others are moving relatively fast. It is common to refer to the average speed of the particles. To gain a feel for what a typical average speed is, consider nitrogen gas (N2) at a temperature of 25°C. The average speed of the N2 molecules is estimated at 516 m/s. Speeds of other gases are shown in the table below.
Gases Have Predictable Behaviors
Gases have many predictable behaviors. These behaviors are often described by statements known as gas laws. The gas laws focus on four main quantities - pressure, volume, temperature, and the number of moles of gas particles. These four quantities describe a sample of gas. A change in one quantity will cause a change in one or more of the other quantities. Gas laws describe the interdependence of these quantities in both a qualitative and a quantitative manner. In Lesson 2, we will discuss these gas laws in great detail. But first, we need to provide some details regarding the meaning of these four quantities.
Before You Leave
- Download our Study Card on The Gaseous State. Save it to a safe location and use it as a review tool.
- Try Activity 1 of our States of Matter Concept Builder. The first activity - Who Am I? - investigates the differences between solids, liquids, and gases.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Identify whether the following statements are True or False regarding a sample of gas? For those statements that are false, correct the statement or explain why it is false.
- It is not possible to apply pressure to a sample of gas.
- The gas inside a small balloon has a volume of 425 mL. If all the gas is emptied into a glass jar, then the gas volume will likely be different.
- Gas particles expand to fill the container because the particles themselves are larger and need more space to fit into the container.
- Solids are more compressible than liquids which are more compressible than gases.
- When a liquid turns to a gas, the mass and the volume of the particular gas will increase many by a large factor.
- Because a gas particle is so small compared to the size of the container, there is not a lot of difference between a gas like H2 and a gas like SF6. They will be about the same size, have the same interparticle distance, have zero interparticle forces, move at the same speed, and have the same density.
2. Refer to the table of speed values for gas particles. Methane (CH4) has a molar mass of 16.0 g/mol. Helium (He) has a molar mass of 4.0 g/mol. Observe their speeds and complete the following sentences.
- Methane has a molar mass that is _________ (two times larger, four times larger, two times smaller, four times smaller) than helium.
- The average speed of a methane particle is _________ (two times larger, four times larger, two times smaller, four times smaller) than the average speed of a helium particle.
- This indicates that the particle speed is ________ (increasing with, decreasing with, not affected by) increasing molar mass.
- This data in the table for methane and helium a quadrupling of the molar mass results in the _______ (quadrupling, doubling, halving, quartering) of the average speed.
3. Besides molar mass, what quantity also affects the speed at which particles of gas move about the container?