Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Ionic Bonding
Part a: Electronegativity and Bond Types
Part 1a: Electronegativity and Bond Types
Part 1b:
Ions and Ionic Bonds
Part 1c:
Ionic Compounds
What is a Bond?
 Atoms of different elements or the same elements can combine to form molecules. Here the word combine means to bond together by means of an electrostatic attraction. Positive charges on one atom attract the negative charges of the other atom … and vice versa. The nature of the attraction can vary depending on the type of atom (i.e., element) involved. But regardless of which elements are involved, the outer shell electrons on the atoms are the negatively charged players in the game of electrostatic attraction. This attraction between atoms is a chemical bond and results in the formation of a molecule. When bonded together, atoms are more energetically stable than when existing as isolated atoms.
Atoms of different elements or the same elements can combine to form molecules. Here the word combine means to bond together by means of an electrostatic attraction. Positive charges on one atom attract the negative charges of the other atom … and vice versa. The nature of the attraction can vary depending on the type of atom (i.e., element) involved. But regardless of which elements are involved, the outer shell electrons on the atoms are the negatively charged players in the game of electrostatic attraction. This attraction between atoms is a chemical bond and results in the formation of a molecule. When bonded together, atoms are more energetically stable than when existing as isolated atoms.
 A variety of representations are used to represent such bonds. Most students are familiar with the ball-and-stick physical model (shown above). We will use other models to represent chemical bonds that rely on representations that you have already been exposed to in this Chemistry Tutorial. These included electron shell diagrams, orbital box diagrams, electron cloud diagrams, and electron configurations. An electron cloud diagram (shown at right) uses shading to represent the probability of an electron being located at a particular location at a moment in time. The electron is less likely to be found at the lightly shaded regions.
A variety of representations are used to represent such bonds. Most students are familiar with the ball-and-stick physical model (shown above). We will use other models to represent chemical bonds that rely on representations that you have already been exposed to in this Chemistry Tutorial. These included electron shell diagrams, orbital box diagrams, electron cloud diagrams, and electron configurations. An electron cloud diagram (shown at right) uses shading to represent the probability of an electron being located at a particular location at a moment in time. The electron is less likely to be found at the lightly shaded regions.
Electron Sharing
 It’s time for a thought experiment. Imagine two isolated hydrogen atoms located some distance apart that begin to approach each other. Each has one proton in the nucleus and one electron in its n=1 electron shell. Let’s refer to the two atoms as A and B. As the atoms approach, the proton of A and electron of B begin to experience an electrostatic attraction. Similarly, the proton of B and electron of A have a similar interaction. Opposites attract! The closer that the atoms approach, the stronger the attractions become. These attractions between A and B cause the otherwise symmetrical electron cloud to become distorted. Electrons demonstrate a higher probability of being located between the two nuclei. The electron clouds of A and B are no longer symmetrically distributed about each atom but rather distributed between the atoms. At a particular separation distance, the electron of A might spend as much time nearer the nucleus of B as it does near the nucleus of A. The same can be said of the electron of B. At this separation distance, we would say that the two electrons are being shared by the two atoms. A bond is formed as a result of the sharing of electrons.
It’s time for a thought experiment. Imagine two isolated hydrogen atoms located some distance apart that begin to approach each other. Each has one proton in the nucleus and one electron in its n=1 electron shell. Let’s refer to the two atoms as A and B. As the atoms approach, the proton of A and electron of B begin to experience an electrostatic attraction. Similarly, the proton of B and electron of A have a similar interaction. Opposites attract! The closer that the atoms approach, the stronger the attractions become. These attractions between A and B cause the otherwise symmetrical electron cloud to become distorted. Electrons demonstrate a higher probability of being located between the two nuclei. The electron clouds of A and B are no longer symmetrically distributed about each atom but rather distributed between the atoms. At a particular separation distance, the electron of A might spend as much time nearer the nucleus of B as it does near the nucleus of A. The same can be said of the electron of B. At this separation distance, we would say that the two electrons are being shared by the two atoms. A bond is formed as a result of the sharing of electrons.
Our thought experiment begs this Why? question:
Why don’t the atoms continue to approach so that the electron of one hydrogen atom gets even closer to the proton of the other hydrogen atom?
Electrostatic interactions involve more than the attractive force between opposite charges. There is also a repulsive force between like charged objects. As the atoms continue to approach, the interaction between the positive protons becomes stronger and stronger. This repulsive interaction is destabilizing. It causes the atoms to be pushed away from each other. There is an optimal distance between nuclei at which the combined effect of attractive and repulsive forces is most stabilizing. We call this distance the
bond distance. The atoms in molecules spend the most amount of time at this optimal distance of separation.
Equal vs. Unequal Sharing
A bond that results from the sharing of electrons is a covalent bond. We will discuss covalent bonds in great detail in Lesson 2. For now, let’s distinguish between two contrasting types of covalent bonds. The above thought experiment involved two identical atoms of hydrogen. There is a slight twist to this discussion when the approaching atoms are hydrogen and oxygen. Hydrogen and oxygen would both offer an electron to be shared between the two of them. The result is a covalent bond. But the shared electrons would not be shared equally. Oxygen has a greater electronegativity than hydrogen. Electronegativity is the measure of the tendency of an atom to attract shared electrons towards itself. Being more electron greedy, oxygen pulls the electron cloud closer to itself. There was an even distribution of shared electrons in the H-H covalent bond. But there is an uneven distribution of shared electrons in the H-O bond. A bond like the H-H bond is classified as a nonpolar covalent bond. A bond like the H-O bond is a polar covalent bond.
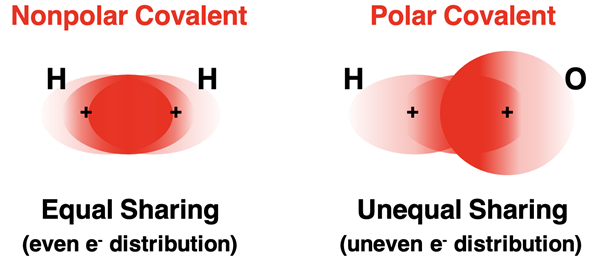
 The polarity of a bond indicates that the center of negative charge is at a different location as the center of positive charge. This is always the case when there is an uneven charge distribution. Since the oxygen nucleus pulls the shared electrons towards itself, the center of negative charge is closer to the oxygen nucleus. Borrowing from the world of electricity, these charge centers are referred to as positive and negative poles. The oxygen atom is said to have a partial negative charge (δ-) and the hydrogen atom has a partial positive charge (δ+). The significance of the term partial charge is that it is not a full -1 or +1 charge but rather a fraction of a full charge. The greater the difference in electronegativity for the two bonded atoms, the more polar that the bond will be and the greater the fraction of charge will be.
The polarity of a bond indicates that the center of negative charge is at a different location as the center of positive charge. This is always the case when there is an uneven charge distribution. Since the oxygen nucleus pulls the shared electrons towards itself, the center of negative charge is closer to the oxygen nucleus. Borrowing from the world of electricity, these charge centers are referred to as positive and negative poles. The oxygen atom is said to have a partial negative charge (δ-) and the hydrogen atom has a partial positive charge (δ+). The significance of the term partial charge is that it is not a full -1 or +1 charge but rather a fraction of a full charge. The greater the difference in electronegativity for the two bonded atoms, the more polar that the bond will be and the greater the fraction of charge will be.
 Electron Transfer
Electron Transfer
The electronegativity value of hydrogen is 2.1. The electronegativity value of oxygen is 3.5. It’s a clear electronegativity (EN) mismatch. But it’s not an extreme EN mismatch. Now let’s consider a sodium atom (EN=0.9) and a fluorine atom (EN=4.0). What happens as these atoms approach?
 Sodium (Na), with a [Ne]3s1 electron configuration, has one valence shell electron. Fluorine (F), with a [He]2s22p5 electron configuration, has seven valence shell electrons. One theme of this chapter is that every atom wants to be like HeNeArKrXeRn. Say it! Remember it! Those are the elemental symbols of the noble gases in periods 1 through 6. Every atom would like to gain, share, or lose electrons in order to acquire the electron configuration of one of these elements.
Sodium (Na), with a [Ne]3s1 electron configuration, has one valence shell electron. Fluorine (F), with a [He]2s22p5 electron configuration, has seven valence shell electrons. One theme of this chapter is that every atom wants to be like HeNeArKrXeRn. Say it! Remember it! Those are the elemental symbols of the noble gases in periods 1 through 6. Every atom would like to gain, share, or lose electrons in order to acquire the electron configuration of one of these elements.
If sodium could rid itself of its one valence electron, it would have the configuration of neon. Sodium’s low electronegativity value is in part due to the fact that it would rather lose an electron to become like neon rather than  gain an electron. Fluorine is one electron short of having the electron configuration of neon. By gaining an electron it has a s2p6 electron configuration, just like that of a noble gas. Fluorine’s high electronegativity value is in part due to the fact that it would love to gain that electron. Do you see the set-up? Sodium has an electron to give. Fluorine has a place to put it. These two atoms are not interested in sharing any electrons. This is the set-up for electron transfer. Sodium transfers an electron to fluorine and empties its n=3 energy level of its one valence electron and becomes like neon. Fluorine, with its high electronegativity exerts a strong pull on that electron and tugs it away from sodium. With the additional electron, fluorine fills all orbitals of its n=2 energy level and becomes like neon. It acquires an s2p6 electron configuration.
gain an electron. Fluorine is one electron short of having the electron configuration of neon. By gaining an electron it has a s2p6 electron configuration, just like that of a noble gas. Fluorine’s high electronegativity value is in part due to the fact that it would love to gain that electron. Do you see the set-up? Sodium has an electron to give. Fluorine has a place to put it. These two atoms are not interested in sharing any electrons. This is the set-up for electron transfer. Sodium transfers an electron to fluorine and empties its n=3 energy level of its one valence electron and becomes like neon. Fluorine, with its high electronegativity exerts a strong pull on that electron and tugs it away from sodium. With the additional electron, fluorine fills all orbitals of its n=2 energy level and becomes like neon. It acquires an s2p6 electron configuration.
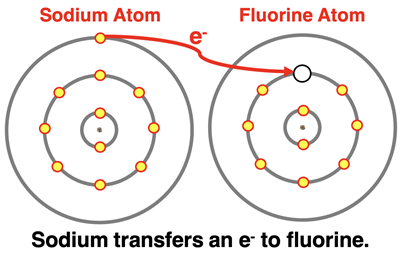
 You might be thinking (and good for you if you are), so what does this have to do with bonding? So what if Na loses an electron and F gains one. Where’s the bond? Awesome question! We mentioned at the onset: a bond is the result of an electrostatic attraction. Sodium loses one valence shell electron and becomes the Na+ ion. Fluorine gains one valence shell electron and becomes the F- ion. These are full charges (1+ and 1-), not partial charges (δ+ and δ-). The transfer of an electron results in the formation of oppositely charged ions. The opposite charges attract with a strong bonding force. A bond that results from the transfer of one or more electrons is an ionic bond.
You might be thinking (and good for you if you are), so what does this have to do with bonding? So what if Na loses an electron and F gains one. Where’s the bond? Awesome question! We mentioned at the onset: a bond is the result of an electrostatic attraction. Sodium loses one valence shell electron and becomes the Na+ ion. Fluorine gains one valence shell electron and becomes the F- ion. These are full charges (1+ and 1-), not partial charges (δ+ and δ-). The transfer of an electron results in the formation of oppositely charged ions. The opposite charges attract with a strong bonding force. A bond that results from the transfer of one or more electrons is an ionic bond.
Classifying Bond Types
In Chapter 2 of this Chemistry Tutorial, we learned that elements can be classified as metals, nonmetals, and metalloids.
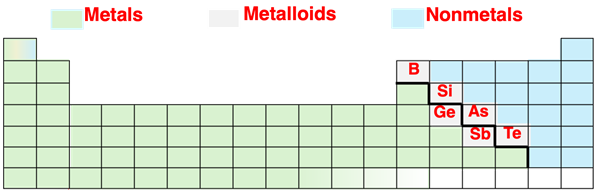
The metals generally have a low electronegativity and four or less valence shell electrons. The nonmetals generally have a relatively high electronegativity and four or more valence shell electrons. A metal and a nonmetal will usually form ionic bonds. The metal will transfer one or more electrons to the nonmetal. Oppositely charged ions form and those ions attract to form a bond. On the other hand, two nonmetals will form covalent bonds by sharing electrons. Two nonmetal atoms with similar or identical electronegativity values will form a nonpolar covalent bond. Polar covalent bonds are formed between two nonmetals with significantly different electronegativity values.

Electronegativity Effects
In Lesson 4d of Chapter 5, we discussed electronegativity values as a periodic trend. The table below displays electronegativity values for groups 1-17 of the periodic table. You will notice that the metallic elements have the lowest electronegativity values and the nonmetallic elements have the highest electronegativity values. Hydrogen is located at the top of the alkali metal group (group 1). It is very different than the alkali metals in many ways. When discussing chemical bonding, it might be better to imagine it being located above boron.
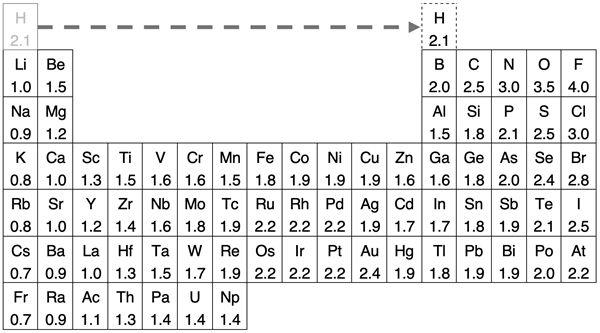
As we have learned, electronegativity is a key factor in predicting the type of bond formed by two atoms. The variable that best predicts the type of bond formed is the difference in electronegativity of the bonding atoms. If we are forced to set cut-off points, then we would suggest that polar covalent bonds form between elements that have electronegativity differences greater than 0.4 and less than 1.7. Nonpolar covalent bonds form between elements with electronegativity differences of 0.4 or less. And ionic bonds form between elements with electronegativity differences greater than or equal to 1.7. The diagram below shows these cut-off points and places a variety of bonds in the various regions.
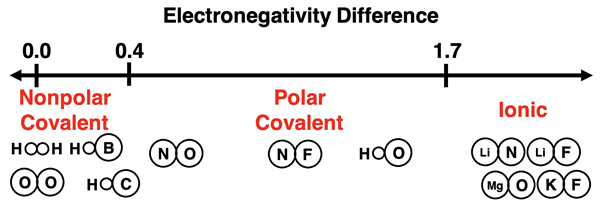
Having cut-off points in electronegativity differences to classify bond types is always problematic. First, there doesn’t seem to be any universally agreed upon values that serve as cut-off points. Second, it often leads to situations in which a metal and a nonmetal get classified as a covalent bond; yet the compound formed by the two elements have all the characteristics of an ionic compound.
 A better approach to chemical bond type is to think in terms of a continuum of varying electronegativity differences without any cut-off points. Two atoms with large electronegativity differences have a high ionic character and a low covalent character. Two atoms with a small or no electronegativity difference have a high covalent character and a low ionic character.
A better approach to chemical bond type is to think in terms of a continuum of varying electronegativity differences without any cut-off points. Two atoms with large electronegativity differences have a high ionic character and a low covalent character. Two atoms with a small or no electronegativity difference have a high covalent character and a low ionic character.
We will discuss ionic bonds in further detail in Lesson 1b. Our detailed discussion of covalent bonds begins in Lesson 2a.
Before You Leave
- Practice. Try our Bond Polarity Concept Builder. It’s great practice!
- More great practice ahead with our Science Reasoning Center activity titled The Periodic Table, Elements, and Their Bonds. Activities 2 through 4 are great follow-ups to Lesson 1a.
- Download our Study Card on Chemical Bond Types. Save it to a safe location and use it as a review tool. (Coming Soon)
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Consider two atoms – Atom A and Atom B. Both are hydrogen atoms. Identify three types of electrostatic interactions between A and B. Identify each type as being either a repulsive interaction or an attractive interaction.
2. Consider the bonds of C (EN=2.5), N (EN=3.0), and O (EN=3.5) bonding with hydrogen (EN=2.1). Match the three bonds C-H, N-H, and O-H to one of the three diagrams below that displays the distortion of the electron cloud.
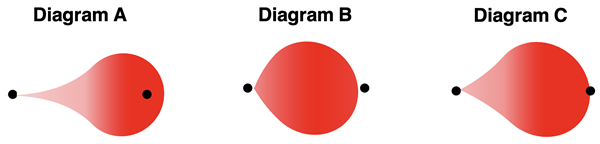
3. Is it possible for two different elements to form a bond that is nonpolar covalent? Or are nonpolar covalent bonds only formed by two identical elements?
4. How many valence electrons are in a noble gas?
5. Without using any electronegativity values, identify the following bonds as being ionic, nonpolar covalent, or polar covalent.
a. C-C
b. Rb-F
c. Mg-O
d. C-O
e. O-O
6. The author states that one problem with having cut-off points for the various types of bonds is that it “leads to situations in which a metal and a nonmetal get classified as a covalent bond.” Use the electronegativity table to identify a metal and a nonmetal that would form a bond that gets classified as a polar covalent bond instead of an ionic bond.
7. Use the table of electronegativity values to rank the following bonds in order of increasing ionic character (from least ionic to most ionic).
F-F
H-F
C-F
K-F
O-F
N-F