Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Properties of Liquids
Part e: Viscosity
Part a:
Boiling and Melting
Part b:
Vapor Pressure
Part c:
Surface Tension
Part d:
Capillary Action
Part e: Viscosity
What is Viscosity?
One of the very obvious properties of a liquid is its ability to flow. Substances in the liquid and gas state are fluids. Fluids flow. Place a solid ice cube in a glass container and the H2O(s) maintains its fixed shape. But when the solid melts and turns to liquid, the H2O(s) will assume the shape of its container. And being a fluid, the H2O(s) can be poured to a different-shaped (or sized) container and it will assume a different shape.
All liquids display this same property of flow. But not all liquids are equal. Some liquids flow readily. Remove the cap from a water bottle and turn it upside down and you will observe a liquid that flows readily. While motor oil in a can or syrup in a bottle will also flow, their flow rate will be noticeably lower. Syrup in particular exhibits a large amount of resistance to flow. The ease at which liquids flow is related to the viscosity of the liquid. Viscosity is a property of a fluid that describes its resistance to flow. You can think of it as the internal resistance. Water has a relatively low resistance and flows readily. Syrup has a high resistance and has more difficulty flowing.


Source: Wikimedia Commons
A Model of Flow
 If a spoon-full of syrup is tipped, it will begin to flow under the influence of gravity. Not every molecule of fluid will flow at the same rate. We can think of the flow as occurring in layers. The layer of molecules on the syrup’s upper surface flow at a different rate than the molecules at the bottom of the spoon. In order to escape the spoon, the uppermost layers of molecules must slide past and away from the layer beneath it. Cohesive forces (i.e., intermolecular forces) between molecules provides a resistance to the movement of one layer past another layer. As molecules in adjacent layers pull upon one another, there is an internal resistance to the flow.
If a spoon-full of syrup is tipped, it will begin to flow under the influence of gravity. Not every molecule of fluid will flow at the same rate. We can think of the flow as occurring in layers. The layer of molecules on the syrup’s upper surface flow at a different rate than the molecules at the bottom of the spoon. In order to escape the spoon, the uppermost layers of molecules must slide past and away from the layer beneath it. Cohesive forces (i.e., intermolecular forces) between molecules provides a resistance to the movement of one layer past another layer. As molecules in adjacent layers pull upon one another, there is an internal resistance to the flow.
Internal resistance as a description of viscosity highlights two aspects of viscosity. First, viscosity is associated with the interaction of fluid particles with one another. It is not the result of the fluid’s interaction with its surroundings. While a fluid can interact with its container, that interaction does not describe viscosity. Viscosity is a property of the fluid itself and not descriptive of how the fluid interacts with its surroundings.
Second, viscosity describes the effort of molecules of the fluid to prevent other molecules from moving. A high viscosity fluid is a fluid whose particles have a strong tendency to impede the flow. This explains why the flow rate and the viscosity are inversely related to one another. A high viscosity fluid flows at a low rate.
Intermolecular Forces and other Variables
Given the above description of viscosity, it is easy to understand how intermolecular forces affect the viscosity. The intermolecular forces of attraction between molecules in adjacent layers heightens the resistance to flow and reduces the flow rate. Substances with the strongest intermolecular forces tend to have the greatest viscosities and are slowest to flow. This is illustrated in the table below for five selected liquids.
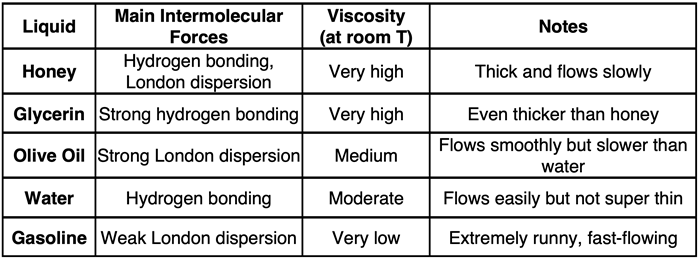
 The liquids above are quite different than one another in terms of atomic composition. A fairer comparison would involve selecting substances that are made of the same elements. Viscosity values for a class of hydrocarbons known as alkanes are widely available. The table below shows the name, formula, and viscosity value for seven alkanes that are liquids at room temperature. All alkanes are structurally similar, consisting of a long chain of carbon atoms. The hydrogen atoms are attached to the C atoms with three H atoms on the ends of the chain and two H atoms on each interior carbon.
The liquids above are quite different than one another in terms of atomic composition. A fairer comparison would involve selecting substances that are made of the same elements. Viscosity values for a class of hydrocarbons known as alkanes are widely available. The table below shows the name, formula, and viscosity value for seven alkanes that are liquids at room temperature. All alkanes are structurally similar, consisting of a long chain of carbon atoms. The hydrogen atoms are attached to the C atoms with three H atoms on the ends of the chain and two H atoms on each interior carbon.
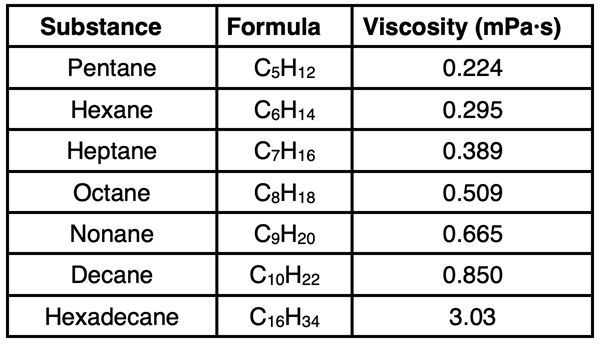
An inspection of the viscosity values show a clear trend: longer chain alkanes have larger viscosity values. All alkanes are nonpolar molecules. The only intermolecular forces experienced by its molecules are London dispersion forces. As we discussed in Lesson 1, London dispersion forces are strongest for larger molecules. Hexadecane, with its 16-carbon chain, experiences considerably larger intermolecular forces than pentane. As such, the viscosity of hexadecane is considerably larger.
 Another variable that affects the viscosity of liquids is the tendency of its molecules to become entangled like cooked spaghetti noodles. This is an especially significant factor for large molecules consisting of hundreds of atoms. These long molecules become entangled as they flow, resulting in a molecular traffic jam. This impedes the movement of one layer of liquid past another layer of liquid. The unusually high viscosity of many substances can be attributed to the tendency of their molecules to become entangled. The high viscosity of slime is due to the entanglement of its lengthy polymer chains.
Another variable that affects the viscosity of liquids is the tendency of its molecules to become entangled like cooked spaghetti noodles. This is an especially significant factor for large molecules consisting of hundreds of atoms. These long molecules become entangled as they flow, resulting in a molecular traffic jam. This impedes the movement of one layer of liquid past another layer of liquid. The unusually high viscosity of many substances can be attributed to the tendency of their molecules to become entangled. The high viscosity of slime is due to the entanglement of its lengthy polymer chains.
Image Credit: Spaghetti Noodles
A Viscosity Experiment
 A relatively common lab experiment on the topic of viscosity involves measuring the time for a steel ball or marble to drop through a column of a liquid. Several test tubes are filled with different liquids. A steel ball with a diameter slightly smaller than the test tube is dropped to the bottom of the test tube. The test tube is stoppered. The test tube is then inverted so that the ball is at the top. The time for the ball to fall back to the bottom is measured.
A relatively common lab experiment on the topic of viscosity involves measuring the time for a steel ball or marble to drop through a column of a liquid. Several test tubes are filled with different liquids. A steel ball with a diameter slightly smaller than the test tube is dropped to the bottom of the test tube. The test tube is stoppered. The test tube is then inverted so that the ball is at the top. The time for the ball to fall back to the bottom is measured.
In order to the ball to fall through the liquid, molecules of the liquid have to flow out of the way and around the ball. For liquids with a high viscosity, this flow can take considerable time and the movement of the ball through the test tube column is very slow. Other liquids with low viscosity quickly flow around the ball and the fall of the ball through the test tube occurs very fast. A few time measurements allow students to rank the liquids according to their viscosity. For the data shown at the right, we would identify liquid B as having the lowest viscosity and liquid C as having the highest viscosity.
Before You Leave
- Download our Study Card on Viscosity. Save it to a safe location and use it as a review tool. (Coming Soon.)
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Complete the following sentences.
The rate at which a liquid flows will ______________ (increase, decrease) with increasing viscosity. Flow rate and viscosity are ______________ (directly, inversely) related. While there can be complicating factors, in general a liquid with strong intermolecular forces will have a ______________ (high, low) viscosity and a ______________ (high, low) flow rate.
2. What is the difference between internal resistance and external resistance?
3. For nonpolar molecules in the same family (e.g., alkanes), one would expect that the _______________ (larger, smaller) molecules will have the greater intermolecular forces, the _______________ (higher, lower) viscosity values, and the _______________ (higher, lower) flow rates.
4. Suppose that the three liquids in the Viscosity Experiment discussed at the end of the lesson are:

- The substance in Test Tube A is likely ____________________.
- The substance in Test Tube B is likely ____________________.
- The substance in Test Tube C is likely ____________________.