Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Properties of Liquids
Part d: Capilliary Action
Part a:
Boiling and Melting
Part b:
Vapor Pressure
Part c:
Surface Tension
Part d: Capillary Action
Part e:
Viscosity
What is Capillary Action?
 Most of us are familiar with the ability of a paper towel to act as a sponge to absorb water from a surface. And most of us are familiar with the workings of a candle - the fibers of the wick pull up melted wax where it is vaporized and combusted. And even in Chemistry class, you have observed the concave meniscus of water in a graduated cylinder or burette. All of these phenomena have one thing in common - capillary action.
Most of us are familiar with the ability of a paper towel to act as a sponge to absorb water from a surface. And most of us are familiar with the workings of a candle - the fibers of the wick pull up melted wax where it is vaporized and combusted. And even in Chemistry class, you have observed the concave meniscus of water in a graduated cylinder or burette. All of these phenomena have one thing in common - capillary action.
Capillary action is the tendency of a liquid to flow through narrow spaces, pores, or narrow columns without the assistance of external forces. Capillary action can be both att ractive and repulsive. It can result in the rise or the sinking of a liquid in the narrow space or column. Capillary attraction accounts for the absorption of water by paper towels, the rise of a liquid upwards through a column, and the passage of water through the tiny pores of an otherwise solid material. The less common capillary repulsion accounts for the occasional formation of a convex meniscus and the tendency of a liquid’s level in an immersed tube to be lower than the level in the surrounding liquid. The distinction between capillary attraction and repulsion is explained with an understanding of two types of forces - adhesion and cohesion.
ractive and repulsive. It can result in the rise or the sinking of a liquid in the narrow space or column. Capillary attraction accounts for the absorption of water by paper towels, the rise of a liquid upwards through a column, and the passage of water through the tiny pores of an otherwise solid material. The less common capillary repulsion accounts for the occasional formation of a convex meniscus and the tendency of a liquid’s level in an immersed tube to be lower than the level in the surrounding liquid. The distinction between capillary attraction and repulsion is explained with an understanding of two types of forces - adhesion and cohesion.
Adhesion vs. Cohesion
 We discussed cohesion forces on the previous page of Lesson 2. Cohesion forces are the forces between particles of the same substance. On the other hand, adhesion forces are the forces between particles of two different substances. A water molecule attracting another water molecule is an example of cohesion. But a water molecule attracting a glass molecule is an example of adhesion. Cohesion forces explained the strong surface tension exhibited by water. Molecules of water stick together. Both cohesion and adhesion forces are the result of interparticle forces - hydrogen bonding, dipole-dipole interactions, and London dispersion forces.
We discussed cohesion forces on the previous page of Lesson 2. Cohesion forces are the forces between particles of the same substance. On the other hand, adhesion forces are the forces between particles of two different substances. A water molecule attracting another water molecule is an example of cohesion. But a water molecule attracting a glass molecule is an example of adhesion. Cohesion forces explained the strong surface tension exhibited by water. Molecules of water stick together. Both cohesion and adhesion forces are the result of interparticle forces - hydrogen bonding, dipole-dipole interactions, and London dispersion forces.
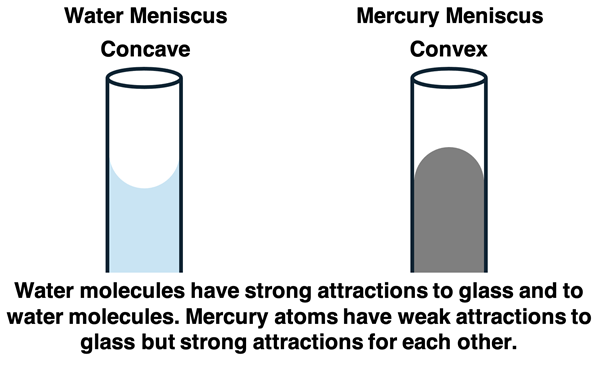
Capillary action is explained by consideration of both cohesion and adhesion forces. This is particularly apparent when you compare the meniscus formed by water and by mercury when placed in a glass column. A meniscus is the curved surface that is observed when a liquid is in contact with another substance. You have likely observed the water meniscus in a graduated cylinder. Water  forms a concave meniscus in a glass cylinder. Glass consists of a collection of randomly oriented SiO2 molecules. Each oxygen atom of an SiO2 molecule has two lone pairs. Water molecules are attracted to these lone pairs by hydrogen bonding. At the edges of the water column, the water molecules are pulled upward by adhesion forces with the glass particles. By rising higher and higher up the glass column, more and more hydrogen bonds can form between the SiO2 molecules of glass and the water molecules (adhesion). Because of cohesive forces, the water molecules stick together. So as water molecules are pulled upward by adhesion to glass, they pull surrounding water molecules with them to form the concave shape.
forms a concave meniscus in a glass cylinder. Glass consists of a collection of randomly oriented SiO2 molecules. Each oxygen atom of an SiO2 molecule has two lone pairs. Water molecules are attracted to these lone pairs by hydrogen bonding. At the edges of the water column, the water molecules are pulled upward by adhesion forces with the glass particles. By rising higher and higher up the glass column, more and more hydrogen bonds can form between the SiO2 molecules of glass and the water molecules (adhesion). Because of cohesive forces, the water molecules stick together. So as water molecules are pulled upward by adhesion to glass, they pull surrounding water molecules with them to form the concave shape.
The meniscus of a mercury column is significantly different than that of water. Because of the large mass of its particles, mercury atoms experience strong London dispersion forces. While the cohesive forces in mercury are very strong, mercury has very weak attraction for the SiO2 molecules of glass. At the edges of the column where mercury touches the glass, the mercury is not pulled upward. Rather, the mercury experiences capillary repulsion and sinks downward at the edges where it is in contact with glass. A convex meniscus is formed. This reduces the number of SiO2-Hg interactions (adhesion forces) while increasing the number of Hg-Hg interactions (cohesion forces).
Capillary Rise in Narrow Tubes
 Suppose that you were to insert three narrow, glass tubes with varying inner diameters into a container filled with water. What would be observed? The water would demonstrate the tendency to flow upward through the narrow tube. Because of the interparticle attractions between water and glass, the water would rise up the narrow column. The height to which the water rises would be affected by the radius of the tubes. As shown, the water rises higher through the narrower column and experiences less rise in the wider column. In fact, the height to which a liquid rises is inversely proportional to the radius of the column.
Suppose that you were to insert three narrow, glass tubes with varying inner diameters into a container filled with water. What would be observed? The water would demonstrate the tendency to flow upward through the narrow tube. Because of the interparticle attractions between water and glass, the water would rise up the narrow column. The height to which the water rises would be affected by the radius of the tubes. As shown, the water rises higher through the narrower column and experiences less rise in the wider column. In fact, the height to which a liquid rises is inversely proportional to the radius of the column.
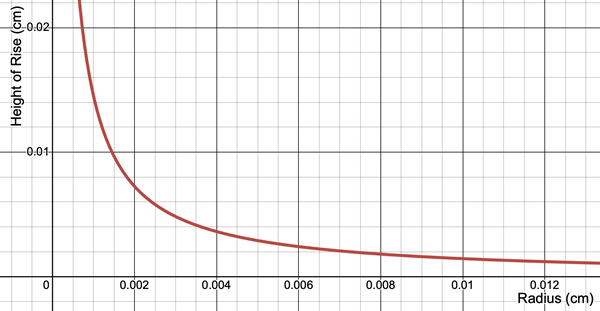
According to Jurin’s Law, the height (h) to which a liquid rises up a capillary column is dependent upon the surface tension of the liquid (γ), the density of the liquid (ρ), the angle of  contact between the liquid (θ), the acceleration of gravity (g), and the radius (R) of the capillary column. A plot of the height as a function of the radius of the capillary column is shown below. The downward sloping line indicates that the height is inversely proportional to the radius of the column.
contact between the liquid (θ), the acceleration of gravity (g), and the radius (R) of the capillary column. A plot of the height as a function of the radius of the capillary column is shown below. The downward sloping line indicates that the height is inversely proportional to the radius of the column.
Porosity and Capillary Action
Capillary action is not limited to the sterile glassware of a chemistry lab - glass tubes, graduated cylinders, and burettes. As already mentioned, capillary action is observed in the wick of a candle as it wicks up melted wax along its fibers. Capillary action is observed in plants and is one of the major mechanisms responsible for photosynthesis. The xylem of plants act as tiny capillary tubes through which water rises upward from the ground to the leaves of the plant where photosynthesis occurs. Even porous materials like paper towels, soil, clay, and concrete allow the flow of water by capillary action, even in an upward direction against the pull of gravity.
 A porous material is a material that is full of miniature holes and openings. Each hole or opening acts as a tiny capillary tube through which a liquid can pass. A sponge is an everyday example of a material that is porous. The miniature pores of a paper towel can also act as channels for water flow. A puddle of water on the kitchen countertop can be quickly absorbed by a sponge or paper towel. Adhesive forces between water molecules and the particles of the sponge or paper towel exert a pull upon water and attract them into and through the openings by capillary action. The next time you have to clean up that aqueous mess in the kitchen, you can be thankful for Chemistry for Better Living.
A porous material is a material that is full of miniature holes and openings. Each hole or opening acts as a tiny capillary tube through which a liquid can pass. A sponge is an everyday example of a material that is porous. The miniature pores of a paper towel can also act as channels for water flow. A puddle of water on the kitchen countertop can be quickly absorbed by a sponge or paper towel. Adhesive forces between water molecules and the particles of the sponge or paper towel exert a pull upon water and attract them into and through the openings by capillary action. The next time you have to clean up that aqueous mess in the kitchen, you can be thankful for Chemistry for Better Living.
It's a surprise to many that concrete is a porous material full of tiny holes and openings. Concrete is a mixture of cement and small solid aggregates like sand and crushed gravel. During construction, the cement and aggregates are mixed; and then water is added. The water reacts with the cement in an exothermic process (heat releasing) known as hydration. As the cement reacts, it serves the function of a glue to bind all the aggregates together into a hardened structure. When the process is complete, the structure consists of a wide, expansive collection of tiny pores and openings. Water can be drawn upwards and sideways through these holes by capillary action.

Source: Wikimedia Commons
Before You Leave
- Download our Study Card on Surface Tension and Capillary Action. Save it to a safe location and use it as a review tool. (Coming Soon.)
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
 1. The Lab apparatus at the right contains water mixed with red food coloring. We all know that “water seeks its own level”. So, what kind of sorcery is this? The level of water in the four tubes is all different. Use what you have learned in this Lesson to explain what is going on.
1. The Lab apparatus at the right contains water mixed with red food coloring. We all know that “water seeks its own level”. So, what kind of sorcery is this? The level of water in the four tubes is all different. Use what you have learned in this Lesson to explain what is going on.
Source: Frey Scientific
2. Mercury added to a glass cylinder forms a convex meniscus. What does this tell you about the relative strength of adhesive and cohesive forces for mercury and glass?
3. Water added to a glass cylinder forms a concave meniscus. What does this tell you about the relative strength of adhesive and cohesive forces for water and glass?
4. Hexane (C
6H
6) is a nonpolar compound consisting of only C-C and C-H bonds. There are no lone pairs of electrons in a hexane molecule. How would you predict the meniscus of hexane compares to the meniscus of water?