Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: The Quantum Mechanical Model
Part b: Orbitals
Part 2a: Schrodinger's Wave Mechanical Model
Part 2b: Orbitals
Part 2c: Energy Levels
Part 2d: Quantum Numbers
 Describing Electrons and Orbitals in Atoms
Describing Electrons and Orbitals in Atoms
The quantum mechanical model describes electrons as being located in regions of space known as orbitals. There are four different orbital types that are important. Each type has a different shape. An s orbital is a spherically shaped orbital. A p orbital is a dumbbell shaped orbital with two lobes on the opposite sides of the nucleus. There are also d orbitals and f orbitals with shapes that are difficult to describe in words.
 Each electron is described by a unique set of four quantum numbers. The first three quantum numbers define the size, shape, orientation, and energy associated with the orbital. The fourth quantum number describes the spin direction of the electron.
Each electron is described by a unique set of four quantum numbers. The first three quantum numbers define the size, shape, orientation, and energy associated with the orbital. The fourth quantum number describes the spin direction of the electron.
Principal Quantum Number
The first quantum number – n – is known as the principal quantum number. It has integer values of 1, 2, 3, etc. The principal quantum number describes the principal energy level of the electron. An electron with an n value of 1 is said to be in the first energy level. An electron with an n value of 2 is said to be in the second energy level. The energy level of the electron increases as the value of n increases. (The second quantum number – l – has a smaller effect upon the energy level.)
 There can be several sets of orbitals having the same n value. The number of types of orbitals for any given energy level is equal to the principal quantum number. The first energy level (n=1) contains only s orbitals. The second energy level (n=2) contains two types of orbitals – s orbitals and p orbitals. The third energy level (n=3) contains three types of orbitals - s orbitals, p orbitals, and d orbitals. And the fourth energy level (n=4) has all four orbital types.
There can be several sets of orbitals having the same n value. The number of types of orbitals for any given energy level is equal to the principal quantum number. The first energy level (n=1) contains only s orbitals. The second energy level (n=2) contains two types of orbitals – s orbitals and p orbitals. The third energy level (n=3) contains three types of orbitals - s orbitals, p orbitals, and d orbitals. And the fourth energy level (n=4) has all four orbital types.
 The principal quantum number also affects the size of the orbitals. An s orbital at the n=1 energy level is smaller than an s orbital at the n=2 energy level. And an s orbital at the n=2 energy level is smaller than an s orbital at the n=3 energy level. This is depicted in the diagram. The same pattern is observed of other orbital types.
The principal quantum number also affects the size of the orbitals. An s orbital at the n=1 energy level is smaller than an s orbital at the n=2 energy level. And an s orbital at the n=2 energy level is smaller than an s orbital at the n=3 energy level. This is depicted in the diagram. The same pattern is observed of other orbital types.
The s Orbital
 As mentioned above, every energy level has an s orbital. The s orbital is a spherical orbital. There is one s orbital at each energy level. It is the lowest energy sublevel for every principal energy level. The s orbital of the first energy level is referred to as the 1s orbital. The s orbital of the second energy level is referred to as the 2s orbital. Preceding the orbital type (“s”) with the principal quantum number (1, 2, 3, etc.) is common notation in quantum mechanics.
As mentioned above, every energy level has an s orbital. The s orbital is a spherical orbital. There is one s orbital at each energy level. It is the lowest energy sublevel for every principal energy level. The s orbital of the first energy level is referred to as the 1s orbital. The s orbital of the second energy level is referred to as the 2s orbital. Preceding the orbital type (“s”) with the principal quantum number (1, 2, 3, etc.) is common notation in quantum mechanics.
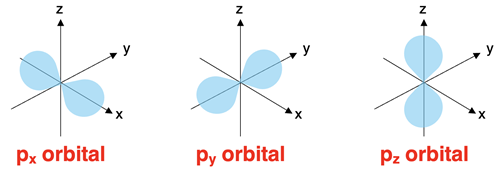
The p Orbitals
The p orbitals are found in all energy levels with a principal quantum number of 2 or higher. The p orbitals are described as dumbbell shaped orbitals. There are two lobes positioned on opposite sides of the nucleus. To help remember the p orbital shape, we call them pinched cylinders (with bulging ends). There are three p orbitals at each of these energy levels. They are distinguished by their orientation relative to the imaginary x-y-z axes and sometimes termed px, py, and pz orbitals. The p orbitals of the second energy level are referred to as the 2p orbitals. The p orbitals of the third energy level are referred to as the 3p orbitals.
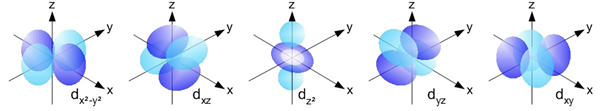
The d Orbitals
The d orbitals are found in all energy levels with a principal quantum number of 3 or higher. There are five d orbitals at each of these energy levels. The shapes of the d orbitals are rather complex. Four of the d orbitals could be described as having a set of four lobes arranged perpendicular to each other. The fifth d orbital looks like a p orbital with a ring around its center. Most introductory Chemistry courses do not require any knowledge of their shapes. The d orbitals of the third energy level are referred to as the 3d orbitals. The d orbitals of the fourth energy level are referred to as the 4d orbitals.
Source: https://commons.wikimedia.org/wiki/File:Single_electron_orbitals.jpg
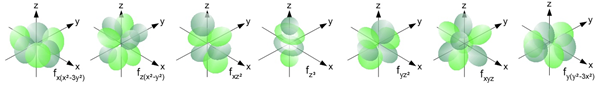
The f Orbitals
The f orbitals are found in all energy levels with a principal quantum number of 4 or higher. There are seven f orbitals at each of these energy levels. Like the d orbitals, their shapes are rather complex and typically not a required understanding in most introductory Chemistry courses. The f orbitals of the fourth energy level are referred to as the 4f orbitals.
Source: https://commons.wikimedia.org/wiki/File:Single_electron_orbitals.jpg
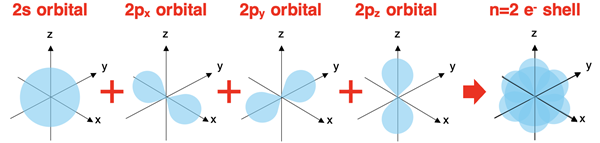
Electron Shells
The collection of orbitals located in each energy level make up what is sometimes referred to as an electron shell. For instance, the second energy level contains both s orbitals and p orbitals. There are three p orbitals with one lying along each axis. These four orbitals (the one s and three p orbitals) combine to form the n=2 electron shell or the second electron shell.
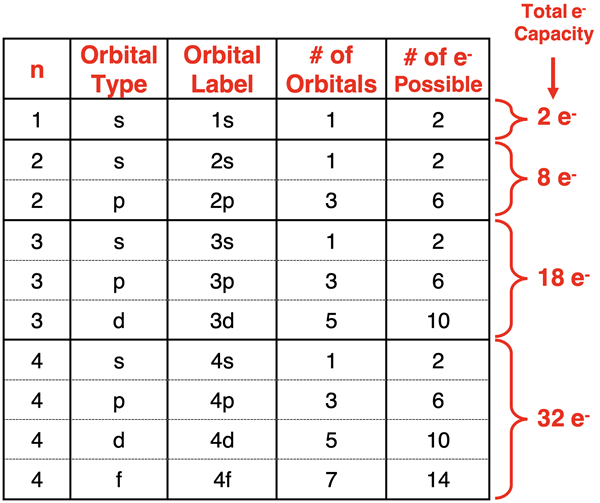
Patterns for the Number of Orbitals and Electron Capacity
As inferred in the above discussion, there are clear patterns that emerge from the mathematical solutions of the Schrodinger equation. These patterns determine what orbital types are present at each energy level, how many of those orbitals are present, and the number of electrons that can be in those orbitals. The patterns are organized into the following table.
Before You Leave
- Download our Study Card on Atomic Orbitals. Save it to a safe location and use it as a review tool.
- Practice. Try our Quantum Mechanics Concept Builder. The first and third of the three activities would make great practice!
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Identify what is wrong with the following statement and make a corrected statement:
As the n value increases, the electron is orbiting with a radius that increases.
2. Identify the following statements as being true or false:
- The n=1 principal energy level has a greater energy than the n=3 principal energy level.
- There are three orbitals present in the n=3 energy level.
- The five 2d orbitals have a higher energy than the three 2p orbitals.
- The 3p orbitals are larger orbitals than the 2p orbitals.
- Every p orbital has the same basic shape regardless of the principal energy level that it is located in.
- There are seven different orbitals in the n=2 electron shell.
- The notation 2p is used when describing an orbital means there are two p orbitals.
- There are a total of four orbitals present in principal energy number of 2.
- Principal energy level 4 consists of four different energy sublevels.
- There are a total of six orbitals present in principal energy level 3.
3. How many types of orbitals have …
- … n=2 quantum numbers?
- … n=3 quantum numbers?
4. How many …
- … p orbitals are there in the n=3 energy level?
- … d orbitals are there in the n=3 energy level?
- … f orbitals are there in the n=3 energy level?
5. What is the maximum number of electrons that can be present in all the ...
- … 2p orbitals?
- … 3d orbitals?