Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Chemistry as a Lab Science
Part c: Experimental Design
Part 1a: Beyond the Scientific Method
Part 1b: Claim-Evidence-Reasoning
Part 1c: Experimental Design
In the previous part of Lesson 1, it was stated that the doing of Chemistry begins with a question and ends with an answer. Chemistry experiments center around the task of answering a testable question. Most experiments that you perform in Chemistry class will be designed by your Chemistry teacher. You may or may not be given procedural steps. But whether you are or are not, it is important to understand the experiment’s design. That is, you need to know what you are doing and why you are doing it. In this part of Lesson 1, we will discuss in general terms the logic that underlies the design of any experiment. If you can understand the principles of experimental design, you are on your way to becoming a stellar do-er of Chemistry.
Identifying a Testable Question
 Not every question is capable of being answered in a high school Chemistry lab. The necessary equipment may not available. Or the procedural steps are too dangerous. Or the required chemicals are too expensive. The question What elements are in moon rocks? is not a testable question for a high school chemistry lab. This question would be testable in a professional lab that had access to moon rocks and the equipment for conducting a chemical analysis. The question How does the density of gold compare to that of silver? is a testable question but it may not be feasible given the high cost of the elements gold and silver. And the question How much radiation does it take to kill a person? is not a testable question whatsoever.
Not every question is capable of being answered in a high school Chemistry lab. The necessary equipment may not available. Or the procedural steps are too dangerous. Or the required chemicals are too expensive. The question What elements are in moon rocks? is not a testable question for a high school chemistry lab. This question would be testable in a professional lab that had access to moon rocks and the equipment for conducting a chemical analysis. The question How does the density of gold compare to that of silver? is a testable question but it may not be feasible given the high cost of the elements gold and silver. And the question How much radiation does it take to kill a person? is not a testable question whatsoever.
Testable questions are those questions for which a student can safely and affordably make observations and measurements that are related to the question. Let’s consider two examples of testable questions:
Testable Question - Example 1
During a unit on the Periodic Table, the teacher poses the question
How are the physical and chemical properties of metallic elements different than those of nonmetallic elements?
What must be considered in order to determine if this question is testable? The following questions would have to be satisfactorily answered in order to design an experiment around this question:
- Are samples of metallic and nonmetallic elements available to experiment with?
- Are these elements safe to work with in a high school Chemistry lab? Are there enough samples of each type of element?
- Are there physical properties that can be tested and observed?
- Are there chemical properties that can be tested and observed?
If the answers to all these questions is Yes, then the question is testable and can become the basis of the experiment.
Testable Question - Example 2
During a unit on Gases, the teacher poses the question
What affect does varying temperature have upon the volume of a sample of gas?
What must be considered in order to determine if this question is testable? The following questions would have to be satisfactorily answered in order to design an experiment around this question:
- Is there a gas available for testing?
- Is there a container with a variable volume that can hold the gas?
- Is there a means of measuring the volume of the gas sample?
- Is there a means of controlling and changing the temperature of the gas and measuring its value?
If the answers to all these questions is Yes, then the question is testable and can become the basis of the experiment.
Procedural Steps
There is typically a collection of steps that must be performed when doing an experiment. Here is a short sampling of steps for an experiment:
- Turn on a hot plate to a medium setting.
- Fill a 500-mL beaker with approximately 200 mL of distilled water.
- Heat the beaker and water to a temperature of 70 °C. Use a digital thermometer to monitor the temperature.
- Using a spatula, weigh boat, and electronic balance, measure out approximately 4.0 g of NaOH pellets; add the pellets to the water.
- Stir with a stirring rod until dissolved.

Steps are ordered. Before a later step can be done, it is likely important that earlier steps are completed. To emphasize the order, the steps might be numbered. Steps include details. The designer of the experiment must give attention to what details are important. Consider step 4 above. Specific tools – spatula, weigh boat, electronic balance – are mentioned. A chemical – NaOH – is identified. An amount of that chemical – 4.0 g – is stated. Whether the amount needed to be exact or approximate is also indicated. And exactly what is to be done with the chemical is described. Details matter since performing the experiment differently could lead to different results.
Procedural steps will always include details about what to observe, measure, and record. These are critical steps as they result in the collection of data. The data eventually becomes the evidence that allows the experimenter to reason towards a claim.
Procedural steps might also describe what to do with the data once it is collected. If numerical information is collected, then the procedure will indicate if any calculations will be performed with it or if any graphs will be constructed. Details about what will be calculated and how data will be graphed are included in the steps. Graphs can be line x-y scatter plots or bar charts or pie charts, etc. The procedure should indicate such details.
Controls and Variables
It is important to give attention to the experimental conditions when designing an experiment. The experiment is usually designed to investigate how one variable affects an outcome. For Example 1, the goal is to determine if being a metal or a nonmetal results in a different set of properties. Suppose one property is the ability to conduct electricity. The procedural steps for determining electrical conductance must be identical for both the metal and the nonmetal. The testing conditions for each must be identical. And there must be consideration of any condition that might interfere with the test. For instance, it would be important that the samples of metal and nonmetals each be dry. Testing wet samples could result in interference since water is a conductor of electricity.

For Example 2 above, the goal is to determine how varying the temperature affects the volume of a sample of gas. Like example 1, it is important to consider all conditions that might interfere with the outcome. Would there be any other variable that might also affect the volume? If so, that variable must be held constant during the experiment. For instance, surrounding air pressure may also affect the volume. It is important to conduct all trials with the same room pressure. Such a condition that is controlled from one trial to the next trial is referred to as a
control. If the Example 2 experiment uses a balloon as the container holding the gas, it may also be important to perform all trials with the same amount of gas inside the balloon.
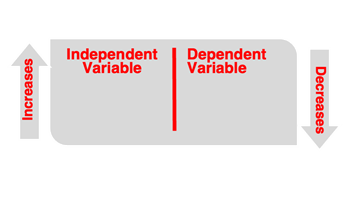
The
variable of an experiment is a measurable quantity or an observable property that is changing. Variables are classified as independent and dependent variables. The
independent variable is the variable that is changed by the experimenter. In Example 1, the dependent variable is the type of element – metal vs. nonmetal. In Example 2, the independent variable is the temperature of the gas. The
dependent variable is the variable that changes as the result of changes made to the conditions of each trial. Values of the dependent variable
depend upon the value of the independent variable.
Looking for Patterns
Chemists are on the constant lookout for
patterns. Most testable questions and resulting experimental designs are intended to discover patterns of behavior in the chemical world. The independent variable is changed, and observations and measurements are made. From the collected data, a chemist may notice that the dependent variable increases as the independent variable increases. Or they may observe the opposite effect. The pattern may be a
proportional pattern. For instance, the dependent variable value may double when the independent variable is doubled. In this case, the dependent variable is
directly proportional to the independent variable. If the dependent variable is
inversely proportional to the independent variable, then the dependent variable will halve as the independent variable is doubled.
Plotting the Path from Question to Answer
There may be times during your Chemistry class in which YOU have to design your own experiment … from beginning to end. Designing an experiment for the first time can be a daunting task. Consider the following steps to assist with the process.
- Brainstorm possible questions.
- Select a question that is testable. Consider safety and cost.
- Identify required equipment for conducting the experiment, including any necessary chemicals.
- Design a step-by-step procedure. Be specific and include details.
- Perform the experiment and collect data – observations and measurements.
- Analyze the data; look for patterns that lead to an answer to the question (a.k.a., a claim)
- State a claim and support it with evidence and reasoning.
The process of designing an experiment requires some creativity. The procedure won’t come to you instantly. You will often need to think outside the box. At times, you may need to do some research to generate some ideas on how to acquire a measurement or control a variable.
Before You Leave ...
- Consider some practice - try the first activity of our Experiments and Variables Concept Builder. It's titled Purpose and Variables.
- And as long as you are there, try the second activity of our Experiments and Variables Concept Builder. It's titled Experimental Design.