Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
 In Lesson 1, it was explained that atoms are the building blocks of matter. Furthermore, it was explained that material objects are made of different types of atoms and combinations of atoms. The presence of different atoms in objects provides different objects with different electrical properties. One such property is known as electron affinity. Simply put, the property of electron affinity refers to the relative amount of love that a material has for electrons. If atoms of a material have a high electron affinity, then that material will have a relatively high love for electrons. This property of electron affinity will be of utmost importance as we explore one of the most common methods of charging - triboelectic charging, also known as charging by friction or rubbing.
In Lesson 1, it was explained that atoms are the building blocks of matter. Furthermore, it was explained that material objects are made of different types of atoms and combinations of atoms. The presence of different atoms in objects provides different objects with different electrical properties. One such property is known as electron affinity. Simply put, the property of electron affinity refers to the relative amount of love that a material has for electrons. If atoms of a material have a high electron affinity, then that material will have a relatively high love for electrons. This property of electron affinity will be of utmost importance as we explore one of the most common methods of charging - triboelectic charging, also known as charging by friction or rubbing.
Suppose that a rubber balloon is rubbed with a sample of animal fur. During the rubbing process, the atoms of the rubber are forced into close proximity with the atoms of the animal fur. The electron clouds of the two types of atoms are pressed together and are brought closer to the nuclei of the other atoms. The protons in the atoms of one material begin to interact with the electrons present on the other material. Amidst the sound of crackling air, you might even be able to hear the atoms saying, "I like your electrons." And of course, the atoms of one material - in this case, the atoms of rubber - are more serious about their claim for electrons. As such, the atoms of rubber begin to take electrons from the atoms of animal fur. When the rubbing has ceased, the two objects have become charged.
The procedure of rubbing a rubber balloon against your hair is quite easily performed. You might try it now if you've never performed it. When done, you will likely notice that the rubber balloon and your  hair will attract each other. On a dry day, you might even be able to let go of the balloon and have it adhere to your hair. (You will also probably notice that the procedure will initiate a bad hair day. Sorry.) This attraction between the two charged objects is evidence that the objects being charged are charged with an opposite type of charge. One is positively charged and the other is negatively charged. How does this happen? How does the simple rubbing together of two objects cause the objects to become charged and charged oppositely?
hair will attract each other. On a dry day, you might even be able to let go of the balloon and have it adhere to your hair. (You will also probably notice that the procedure will initiate a bad hair day. Sorry.) This attraction between the two charged objects is evidence that the objects being charged are charged with an opposite type of charge. One is positively charged and the other is negatively charged. How does this happen? How does the simple rubbing together of two objects cause the objects to become charged and charged oppositely?
How Triboelectric Charging Works
The triboelectic charging process (a.k.a., charging by friction) results in a transfer of electrons between the two objects that are rubbed together. Rubber has a much greater attraction for electrons than animal fur. As a result, the atoms of rubber pull electrons from the atoms of animal fur, leaving both objects with an imbalance of charge. The rubber balloon has an excess of electrons and the animal fur has a shortage of electrons. Having an excess of electrons, the rubber balloon is charged negatively. Similarly, the shortage of electrons on the animal fur leaves it with a positive charge. The two objects have become charged with opposite types of charges as a result of the transfer of electrons from the least electron-loving material to the most electron-loving material.
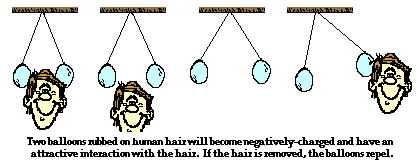
Triboelectic charging is often demonstrated in Physics class. Two rubber balloons can be suspended from the ceiling and hung at approximately head height. When rubbed upon a teacher's head, the balloons became charged as electrons are transferred from the teacher's fur to the balloons. Since the teacher's fur lost electrons, it became positively charged and the subsequent attraction between the two rubbed objects could be observed. Of course, when the teacher pulls away from the balloons, the balloons experienced a repulsive interaction for each other.
 As mentioned, different materials have different affinities for electrons. By rubbing a variety of materials against each other and testing their resulting interaction with objects of known charge, the tested materials can be ordered according to their affinity for electrons. Such an ordering of substances is known as a triboelectric series. One such ordering for several materials is shown in the table at the right. Materials shown highest on the table tend to have a greater affinity for electrons than those below it. Subsequently, when any two materials in the table are rubbed together, the one that is higher can be expected to pull electrons from the material that is lower. As such, the materials highest on the table will have the greatest tendency to acquire the negative charge. Those below it would become positively charged.
As mentioned, different materials have different affinities for electrons. By rubbing a variety of materials against each other and testing their resulting interaction with objects of known charge, the tested materials can be ordered according to their affinity for electrons. Such an ordering of substances is known as a triboelectric series. One such ordering for several materials is shown in the table at the right. Materials shown highest on the table tend to have a greater affinity for electrons than those below it. Subsequently, when any two materials in the table are rubbed together, the one that is higher can be expected to pull electrons from the material that is lower. As such, the materials highest on the table will have the greatest tendency to acquire the negative charge. Those below it would become positively charged.
The Law of Conservation of Charge
The triboelectic charging process (as well as any charging process) involves a transfer of electrons between two objects. Charge is not created from nothing. The appearance of negative charge upon a rubber balloon is merely the result of its acquisition of electrons. And these electrons must come from somewhere; in this case, from the object it was rubbed against. Electrons are transferred in any charging process. In the case of triboelectic charging, they are transferred between the two objects being rubbed together. Prior to the charging, both objects are electrically neutral. The net charge of the system is 0 units. After the charging process, the more electron-loving object may acquire a charge of -12 units; the other object acquires a charge of +12 units. Overall, the system of two objects has a net charge of 0 units. Whenever a quantity like charge (or momentum or energy or matter) is observed to be the same prior to and after the completion of a given process, we say that the quantity is conserved. Charge is always conserved. When all objects involved are considered prior to and after a given process, we notice that the total amount of charge amidst the objects is the same before the process starts as it is after the process ends. This is referred to as the law of conservation of charge.
Flickr Physics Photo
TOP ROW: A plastic tube is charged by rubbing it with synthetic animal fur.
BOTTOM LEFT: The charged tube is then brought near a collection of neutral paper bits at rest on the table.
BOTTOM RIGHT: The charged tube and neutral bits of paper attract each other. The attraction lifts the paper bits off the table.

We Would Like to Suggest ...

Sometimes it isn't enough to just read about it. You have to interact with it! And that's exactly what you do when you use one of The Physics Classroom's Interactives. We would like to suggest that you combine the reading of this page with the use of our
Charging Interactive. You can find it in the Physics Interactives section of our website. The
Charging Interactive is an electrostatics "playground" that allows a learner to investigate a variety of concepts related to charge, charge interactions, charging processes, and grounding. Once you get the hang of the concepts, put your game-face on tap the Play button.
Check Your Understanding
Use your understanding of charge to answer the following questions. When finished, click the button to view the answers.
1. During a physics lab, a plastic strip was rubbed with cotton and became positively charged. The correct explanation for why the plastic strip becomes positively charged is that ...
a. the plastic strip acquired extra protons from the cotton.
b. the plastic strip acquired extra protons during the charging process.
c. protons were created as the result of the charging process.
d. the plastic strip lost electrons to the cotton during the charging process.
2. Saran Wrap has a larger electron affinity than Nylon. If Nylon is rubbed against Saran Wrap, which would end up with the excess negative charge? ____________ Explain.
3. A physics teacher rubs a glass object and a felt cloth together and the glass becomes positively charged. Which of the following statements are true? Circle all that apply.
a. The glass gained protons during the rubbing process.
b. The felt became charged negatively during this rubbing process.
c. Charge is created during the rubbing process; it is grabbed by the more charge-hungry object.
d. If the glass acquired a charge of +5 units, then the felt acquires a charge of -5 units.
e. This event violates the law of conservation of charge.
f. Electrons are transferred from glass to felt; protons are transferred from felt to glass.
g. Once charged in this manner, the glass object and the felt cloth should attract each other.
h. In general, glass materials must have a greater affinity for electrons than felt materials.
4. Which statement best explains why a rubber rod becomes negatively charged when rubbed with fur?
a. The rubber that the rod is made of is a better insulator than fur.
b. The fur is a better insulator than the rubber.
c. Molecules in the rubber rod have a stronger attraction for electrons than the molecules in the fur.
d. Molecules in the fur have a stronger attraction for electrons than the molecules in the rubber rod.