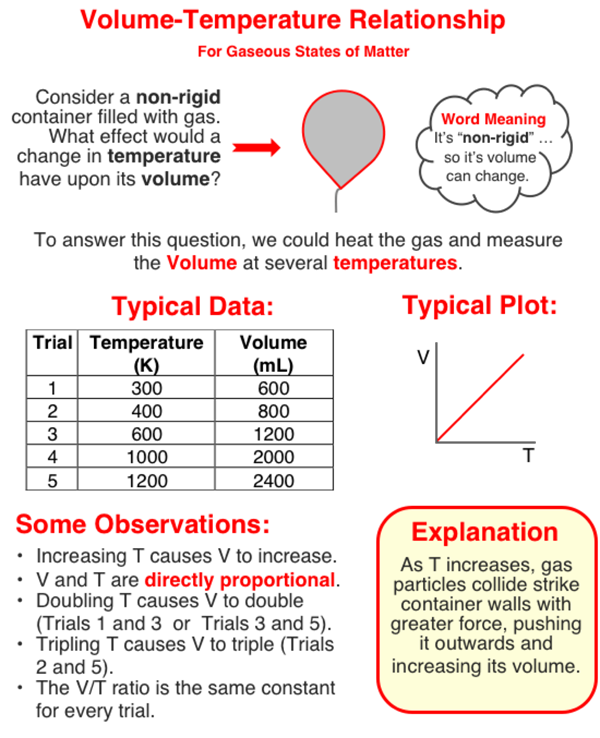
In this question, you need to analyze a set of data to determine the volume when the temperature is 2000 K. One way to do that is to use proportional reasoning. We'll explain that in a moment. But first, take some time to study (and/or scan) the graphic in order to gain familiarity with the volume-temperature relationship. Then continue to the text that continues below the graphic.
Reasoning Proportionally with Volume and Temperature
The temperature of 2000 K is four times the temperature of 500 K. And since four times the Kelvin temperature leads to four times the volume, we should be able to use this four times factor to find the answer. Trial 2 of the table has a temperature of 500 K. So the volume at 2000 K will be four times the volume of Trial 2. So your answer can quickly be found by multiplying the volume at 500 K by four.