In this question, you need to analyze a set of data to determine the effect that a doubling of Kelvin temperature has upon the volume of a gas (held at a constant pressure). The graphic below will provide some background to the topic. Study (and/or scan) the graphic and then return to the text that continues below the graphic.
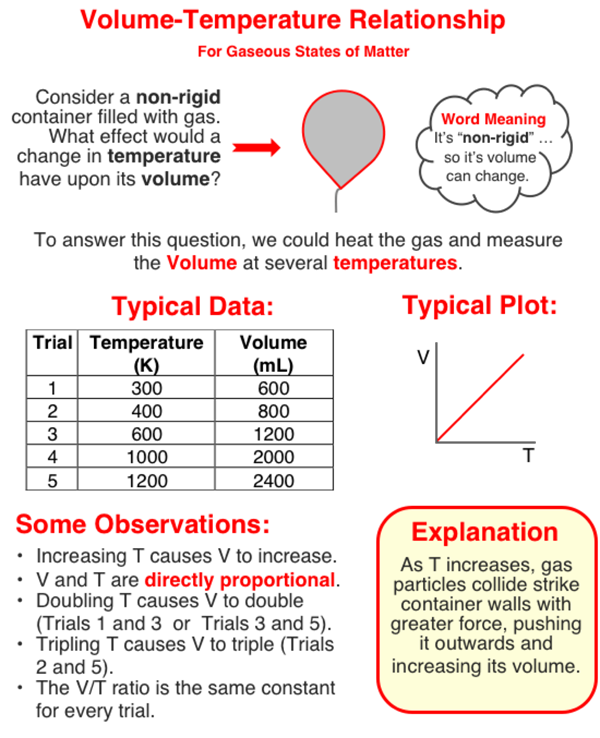
Doubling Kelvin Temperature
A doubling of temperature occurs between two trials whenever the value of temperature in one trial is two times that of another trial. In the graphic directly above, Trials 1 and 3 are examples of doubling because Trial 3 has a temperature (600 K) which is two times that of Trial 1 (300 K). The same can be said of Trials 3 and 5.
So to determine the effect that this doubling has, you will have to look at the corresponding volume values for these two rows. For instance, the volume values in the graphic above for Trials 1 and 3 are 600 mL and 1200 mL. Trial 3 's volume value is two times the value of Trial 1. This indicates that a doubling of the Kelvin temperature leads to a doubling of the volume of the gas.
You will need to apply the same type of thinking to the analysis of the Data Set provided in your question. Good luck.