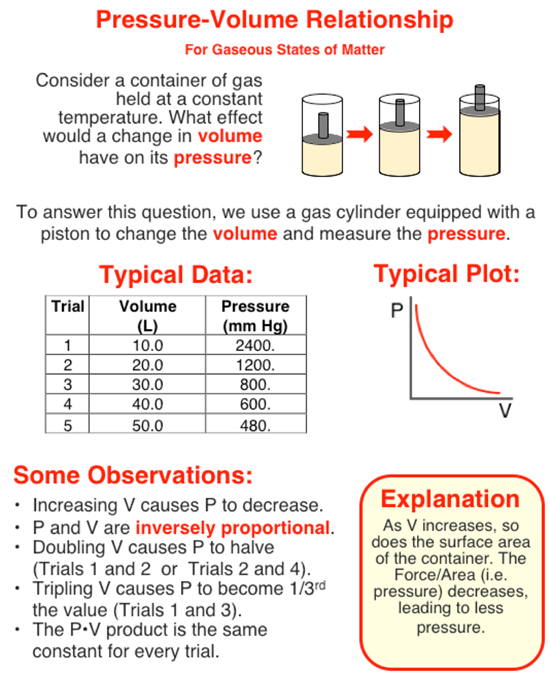
In this question, you need to analyze a set of data to determine the pressure when the volume is 36.0 L. One way to do that is to use proportional reasoning. We'll explain that in a moment. But first, take some time to study (and/or scan) the graphic in order to gain familiarity with the pressure-volume relationship. Then continue to the text that continues below the graphic.
Reasoning Proportionally with Pressure and Volume
The volume of 36.0 L is three times the volume of 12.0 L. And since a tripling of the volume leads to one-third the pressure, we should be able to use this one-third factor to find the answer. Trial 2 of the table has a volume of 12.0 L. So the pressure at 36.0 L will be one-third the pressure listed in Trial 2. So your answer can quickly be found by one-thirding (dividing by 3.0) the Trial 2 pressure value.