In this question, you need to analyze a set of data to determine the effect that a tripling of Kelvin temperature has upon the pressure of a gas (held at a constant volume). The graphic below will provide some background to the topic. Study (and/or scan) the graphic and then return to the text that continues below the graphic.
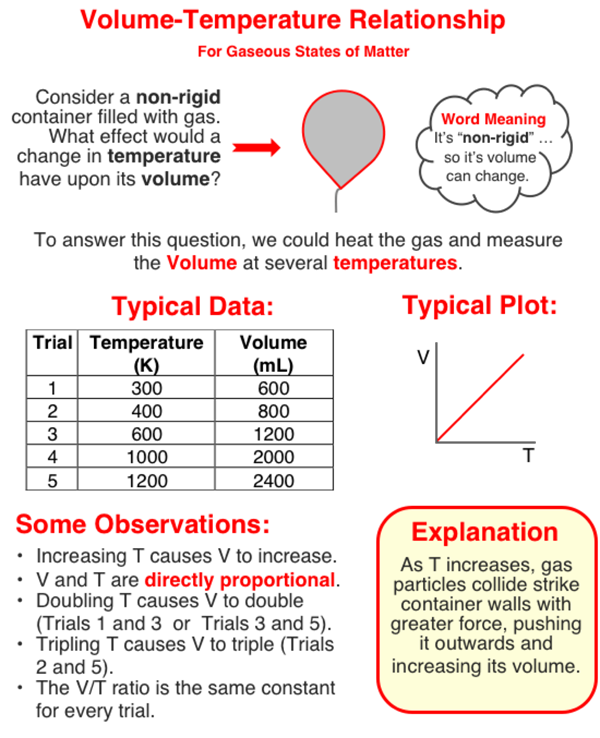
Tripling Kelvin Temperature
A tripling of temperature occurs between two trials whenever the value of temperature in one trial is three times that of another trial. In the graphic directly above, Trials 2 and 5 are examples of tripling because Trial 5 has a temperature (1200 K) which is tree times that of Trial 2 (400 K).
So to determine the effect that this tripling has, you will have to look at the corresponding pressure values for these two rows. For instance, the pressure values in the graphic above for Trials 2 and 5 are 800 mm Hg and 2400 mm Hg. Trial 5 's pressure value is three times the value of Trial 2. This indicates that a tripling of the Kelvin temperature leads to a tripling of the pressure of the gas.
You will need to apply the same type of thinking to the analysis of the Data Set provided in your question. Good luck.