The molar mass of a compound indicates the mass in grams of all the elements in that compound on a per mole basis.
Getting your Trinity Audio player ready...
Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Molar Mass - helpMaster
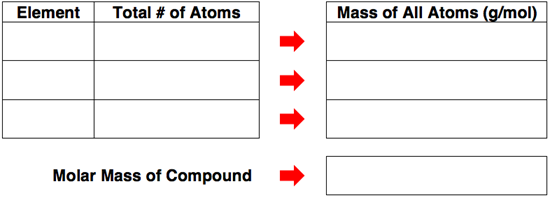
There are four groups of three questions (12 questions in all) in the second activity, titled Calculating Molar Mass. Each question involves the same task. A chemical formula for a compound is given and the student must count the number of atoms of each element and then calculate the molar mass. An example question is shown below.
Version 1:
Use the structure of the following table to determine the molar mass of the compound Ba3(PO4)2.