Density is a physical property of every object that describes the mass per volume ratio. Using information about the mass (amount of stuff) and the volume (the amount of space) for two or more objects, one can make comparisions of their relatice densities.
Getting your Trinity Audio player ready...
Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Density Ranking Tasks - help15
There are three versions of this question. Here is one of the versions.
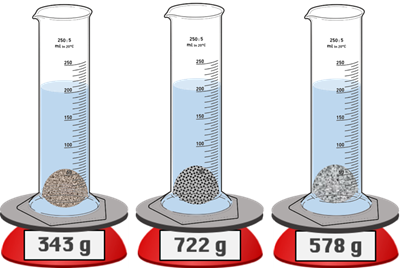
Version 1: A graduated cylinder is filled with 100.0 mL of water. The cylinder is placed on a mass balance and tared (zeroed). In three different studies, samples of solid are added to the cylinder. The mass of solid and the new volume reading are shown. Rank the samples according to their density.
A graduated cylinder is filled with 100.0 mL of water. The cylinder is placed on a mass balance and tared (zeroed). In three different studies, samples of solid are added to the cylinder. The mass of solid and the new volume reading are shown. Rank the samples according to their density.