A chemical reaction is characterized by the change of reactant chemicals (or simply reactants) into product chemicals (or products). The rate at which this change takes places is called the reaction rate. Some reactions occur slowly - like the rusting of the steel body of an automobile. This is a low rate of reaction. Other reactons - like the combustion (i.e., explosion) of hydrogen gas. Hydrogen combustion is a reaction that occurs at a high rate.
Reaction rates can be altered and controlled by changing a few variables. Some variables affecting the rate of a reaction are concentration of aqueous-state reactants, temperature at which the reaction occurs, the exposed surface area of solid-state reactants, and whether or not a catalyst is used.
Getting your Trinity Audio player ready...
Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Collision Model of Reaction Rates - help3
There are three questions in this Question Group. Here is one of them:
Version 1:
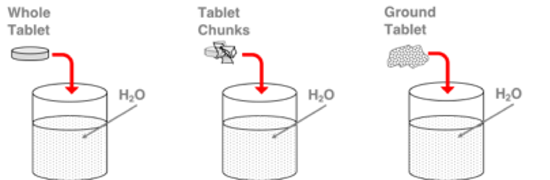
As an Alka Seltzer® dissolves in water, a reaction occurs to produce carbon dioxide gas. Three trials are conducted with a single tablet in a beaker of 300 mL of water. The dependent variable between trials is the degree to which the tablet is ground up - whole tablet (unground), several chunks, and finely ground. Rank the three trials in order of increasing rate of carbon dioxide gas production.