Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: A Framework for Thinking Stoichiometrically
Part b: The Law of Conservation of Mass
Part a:
Recipes, Ratios, and Relationships
Part b: The Law of Conservation of Mass
Part c:
Conversions and Connections
Finding Meaning in the Numbers
 As you continue your studies in science, you will find numerous concepts that cut across all courses. One of those cross-cutting concepts is that there is meaning in the numbers. More often than not, calculations are not ends in themselves. Calculations are pathways towards new understandings. It is the goal of scientists (and science students) to search for patterns amidst the unending stream of numbers.
As you continue your studies in science, you will find numerous concepts that cut across all courses. One of those cross-cutting concepts is that there is meaning in the numbers. More often than not, calculations are not ends in themselves. Calculations are pathways towards new understandings. It is the goal of scientists (and science students) to search for patterns amidst the unending stream of numbers.
On the previous page, we learned how to perform relatively simple calculations to relate the amounts of reactants and products for the ammonia synthesis reaction. The relationships were mole relationships and mass relationships. We used tables to organize our calculated data. Here is an example of one such set of mass calculations from Example 1 on the previous page:

These numbers emerged as we focused on how the mass of a reactant is related to the mass of another reactant and to the mass of a product. Our focus was on learning a skill. In Lesson 1b, we will look at the stream of numbers we generated to find one BIG pattern. Before you move forward to the Mass Analysis section, put on your detective cap and see if you can find the pattern in the table above. HINT: look across a row and not down a column.
 Mass Analysis - Part 1
Mass Analysis - Part 1
Let’s analyze a few rows of the above table, beginning with Row a. Picture the reactants N2 and H2 as being the initial ingredients entering some large reaction vessel. Picture NH3 as being the final product of the reaction process. What we wish to do in our analysis is to compare the initial mass of all reactants to the final mass of the product. We assume in our analysis that all of the reactants are reacted and there is none left over.
In Row a, the total mass of both reactants is 68 g. And the mass of the only product is 68 g. The data show that the initial mass of reactants is equal to the final mass of the products.
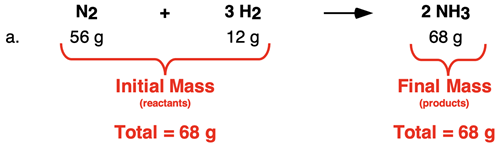
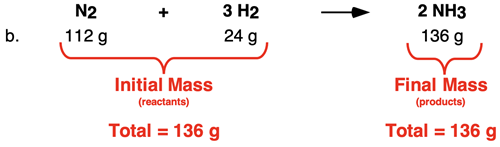
In Row b, the total mass of both reactants is 136 g. And the mass of the only product is 136 g. The data show that the initial mass of both reactants summed together is equal to the final mass of the one product.
And finally, let’s look at Row d. The total mass of both reactants is 51 g. And the mass of the product is also 51 g. The data show that the initial mass (of the reactants) is equal to the final mass (that of the product).
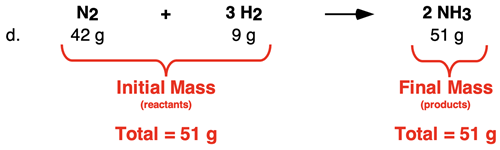
The data show a clear principle: the initial total mass is equal to the final mass. But perhaps this is just a coincidence that only holds true for this reaction or only for this type of synthesis reaction.
Mass Analysis - Part 2
In the interest of being thorough, let’s consider a second reaction of a different type – the combustion of methane. The data below were generated from calculations in Question #5 of the Check Your Understanding section of the previous page. We will use the data to test our hypothesis that the total initial mass equals the total final mass.
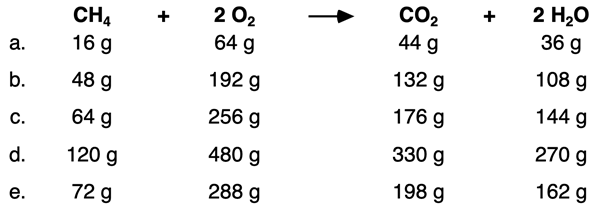
Let’s begin with an analysis of Row a. The total mass of both reactants is 80 g. And the total mass of the two products is 80 g. Once again, the data show that the initial mass of reactants is equal to the final mass of the products.
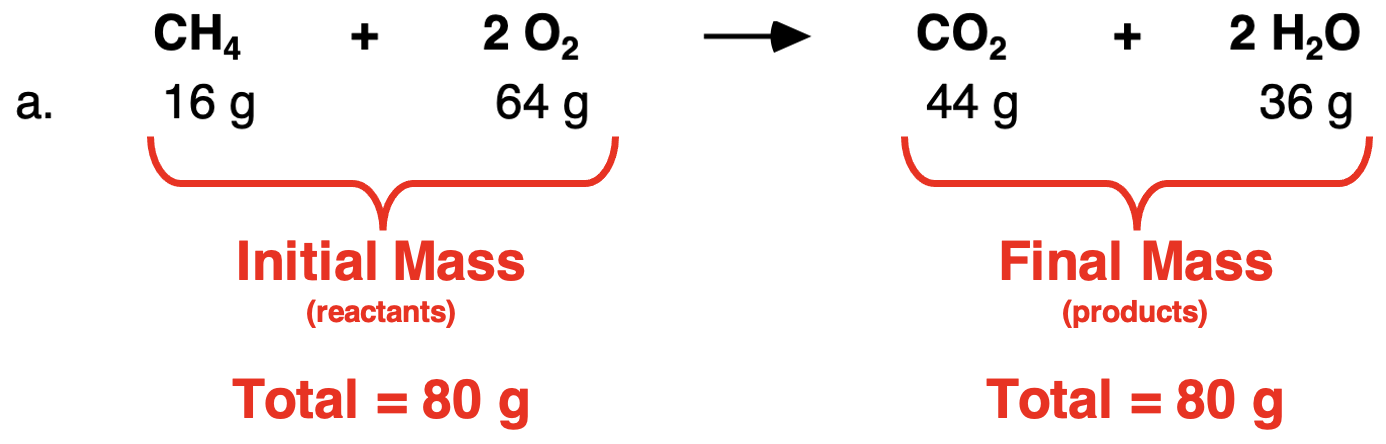
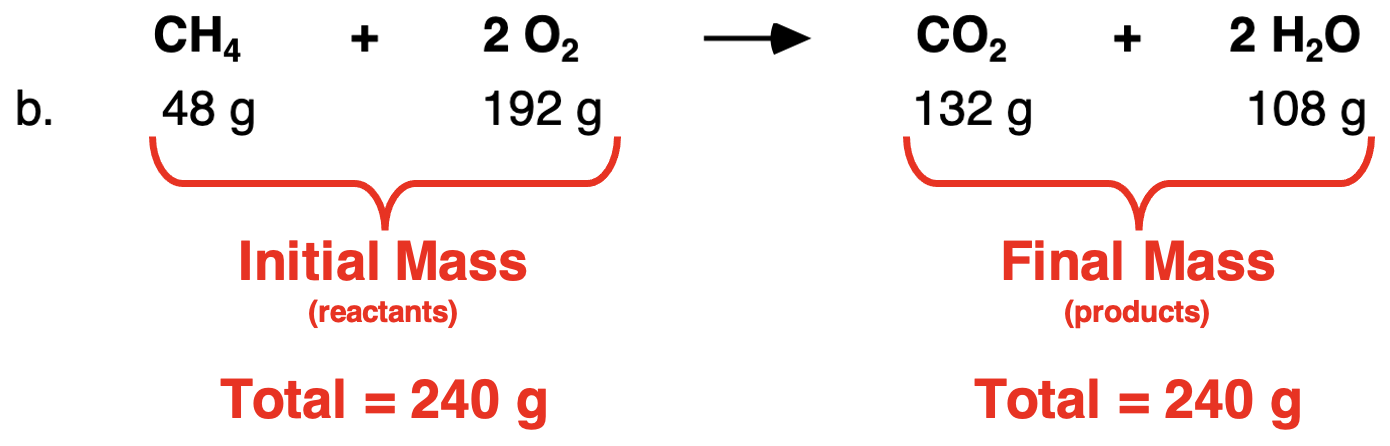
In Row b, the total mass of both reactants is 240 g. And the total mass of both products is 240 g. The initial mass of reactants is equal to the final mass of the products.
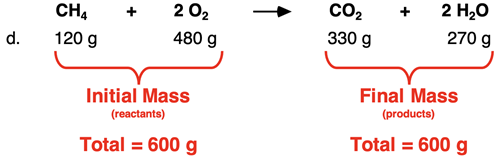
And finally in Row d, the total mass of reactants and the total mass of products each sum to 600 g.
The pattern is clear:
In a chemical reaction, the total mass of all reactants is equal to the total mass of all products.
Law of Conservation of Mass
The principle depicted in the Mass Analysis section is known as the law of conservation of mass. Stated simply, mass is neither created nor destroyed in a chemical reaction. If the parts of an isolated system undergo a reaction, there is neither an increase nor a decrease in the mass of the system as the result of the reaction. If one part of the system undergoes a decrease in mass as the result of a reaction, another part of the system undergoes an increase in mass as the result of the reaction. The decrease in mass of the reactants equals the increase in the mass of the products.
Conservation of mass is more than a theoretical statement. There is plenty of evidence from laboratory studies that total mass is conserved. Insofar as the reactants and products can be contained and measured, one observes that the mass of the container does not change over the course of the reaction. The important experimental condition is that the reactants and products must be contained. If a gas is produced by the reaction, then it must be contained in the container whose mass is being measured.
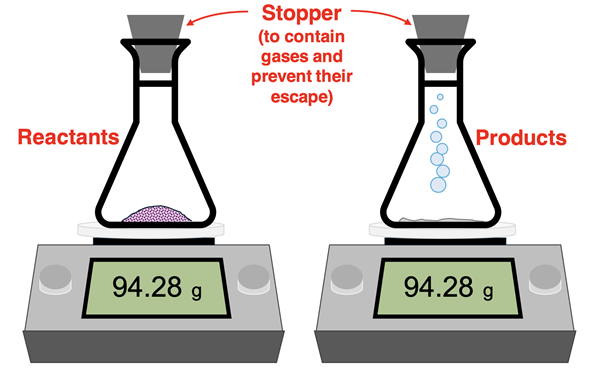
The law of conservation of mass was first proposed by French chemist Antoine Lavoisier in 1774. Lavoisier asserted that the mass of each element involved in a reaction is the same before and after the reaction. Lavoisier’s findings was one of the motivations for John Dalton’s atomic theory.
Particle Explanation of Mass Conservation
In Chapter 8 of this Chemistry Tutorial, we discussed the nature of a chemical reaction. Chemical reactions involve the breaking of chemical bonds that hold atoms together. The atoms re-arrange and then form new bonds, resulting in new chemicals. The reactant chemicals are changed into product chemicals.
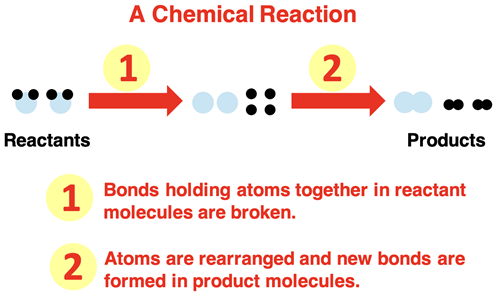
When chemical equations that describe such reactions are written, they are written to be balanced. That is, they are written to communicate that the number of atoms of an element on the reactant side equals the number of atoms of that element on the product side. Atoms are not created nor destroyed in chemical reactions; they are only re-arranged.
The mass of a reactant or product can be attributed to the atoms that it is composed of. If such atoms are not being created nor destroyed, then it makes sense to claim that mass will not be created not destroyed. The law of conservation of mass finds its particle foundation in the fact that chemical reactions involve the rearrangement of atoms and the conservation of atoms. Because atoms are conserved, so is mass.
Before You Leave
- Download our Study Card on Conservation of Mass. Save it to a safe location and use it as a review tool.
- Try our Stoichiometry Science Reasoning Center activity. The activity consists of five parts that center around the concept of atom and mass conservation.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Based on what you learned about the law of conservation of mass, identify the following statements as being either True or False.
- The mass of a reactant never changes. It is the same before and after the reaction.
- The mass of a product before a reaction is equal to the mass of the same product after the reaction.
- The mass of an atom of a given element is the same before the reaction as it is after the reaction.
- The mass of all H atoms in all the reactants is equal to the mass of all H atoms in all the products (assuming the reactants and products contain H atoms).
- The number of moles of reactants must be equal to the number of moles of products.
- If all the reactants do not entirely react, then the law of conservation of mass does not hold true.
2. In the synthesis of water from its elements, hydrogen gas reacts with oxygen gas. If 6 g of hydrogen react with 48 g of oxygen, then how many grams of water will be produced?
3. In the synthesis of water from its elements, hydrogen gas reacts with oxygen gas. If 8 g of hydrogen react with oxygen to produce 72 g of water, then how many grams of oxygen were reacted?