When an electron in an excited state energy level jumps or transitions downward to a lower energy level, it emits electromagnetic radiation ("light") with a given energy. The energy value is equal to the difference in energy between the two energy levels. If the energy falls within the range of the visible light spectrum, then a line will be visible on the spectrum for that element. The color of the line is related to the energy change of the electron.
Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Line Spectra - help7
There are four questions in this Question Group. Each question is very similar to one another. The question below is one of the questions.
Version 1: The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, green, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, green, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E2: ___________
e-Transition from E2 to E1: ___________
e-Transition from E2 to Ground: ___________
Finally, the transition from E3 to Ground state would not be visible since it would correspond to the emission of ...
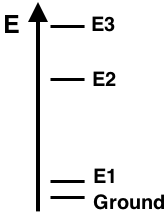 The energy of the emitted photon depends upon the difference in energy between the two states from and to which the electron transitioned. An energy level diagram depicts the relative energy values of the ground state and excited states. As can be seen, the energy differences vary depending on the initial and final state of the electron transition. For instance, the transition from E1 to the Gound state is a low energy transition and would result in the release of a low energy photon. On the other hand, the transition from E3 to the Ground state is a high energy transition and would result in the release of a high energy photon. The diagram allows you to compare the relative energies of a photon released for any given energy level transition.
The energy of the emitted photon depends upon the difference in energy between the two states from and to which the electron transitioned. An energy level diagram depicts the relative energy values of the ground state and excited states. As can be seen, the energy differences vary depending on the initial and final state of the electron transition. For instance, the transition from E1 to the Gound state is a low energy transition and would result in the release of a low energy photon. On the other hand, the transition from E3 to the Ground state is a high energy transition and would result in the release of a high energy photon. The diagram allows you to compare the relative energies of a photon released for any given energy level transition.