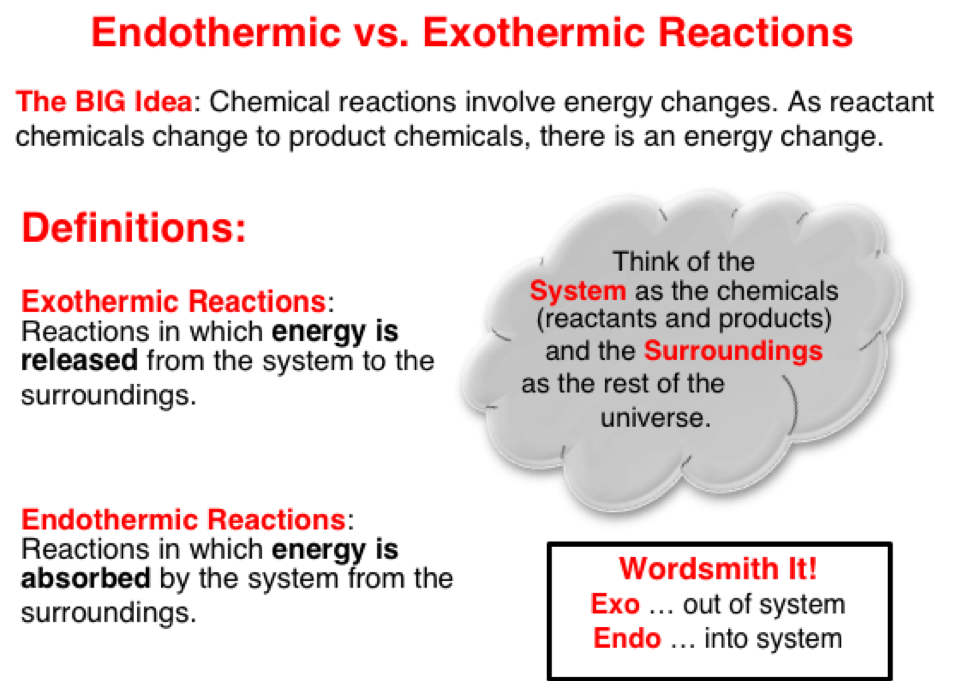
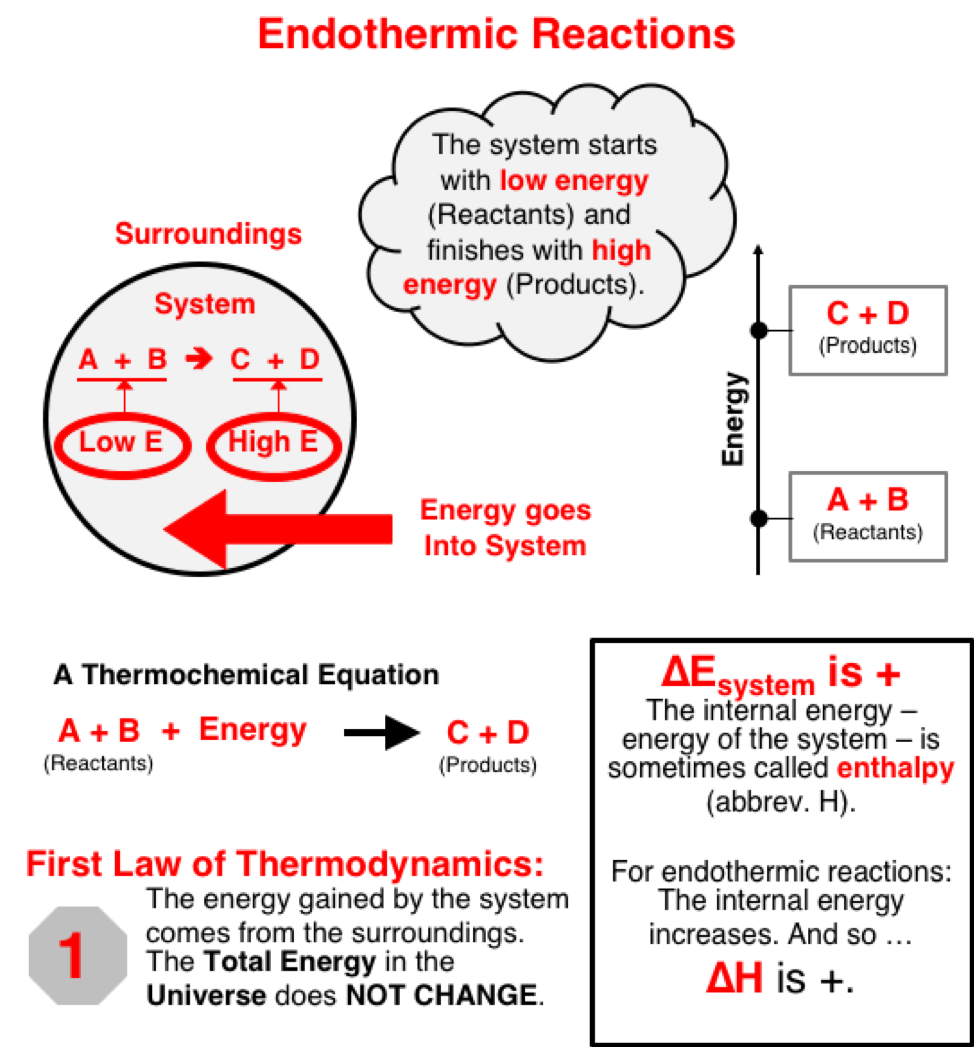
Chemical changes are always accompanied by energy changes. The energy level of reactant chemicals is different than the energy level of product chemicals. The chemicals, often regarded as the system, will acquire energy from the surroundings or release energy to the surroundings as the change from reactants to products take place. This causes a decrease or increase in the temperature of the surroundings.
Getting your Trinity Audio player ready...
Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Which One Doesn't Belong? - Energy and Chemical Reactions - help12
There are three questions in this Question Group. Each question is very similar to one another. The question below is one of the questions.
Version 1:
One of these representations is not like the others. Which one doesn't belong?